Featured Article

Understanding cold storage best practices can help laboratory managers ensure that their medical inventory is in compliance and that they have the documentation to prove it. Guidelines on proper cold chain management for immunobiologics are provided by the Centers for Disease Control and Prevention (CDC).1 The cold chain (see Figure 1) illustrates the importance of keeping vaccines and other pharmaceuticals at a consistently cold temperature—from manufacturing through distribution and storage to end use. Proper storage of pharmaceuticals before they reach the consumer is critical, because any loss of potency during this period may influence safety and efficacy2 and compromise research results.
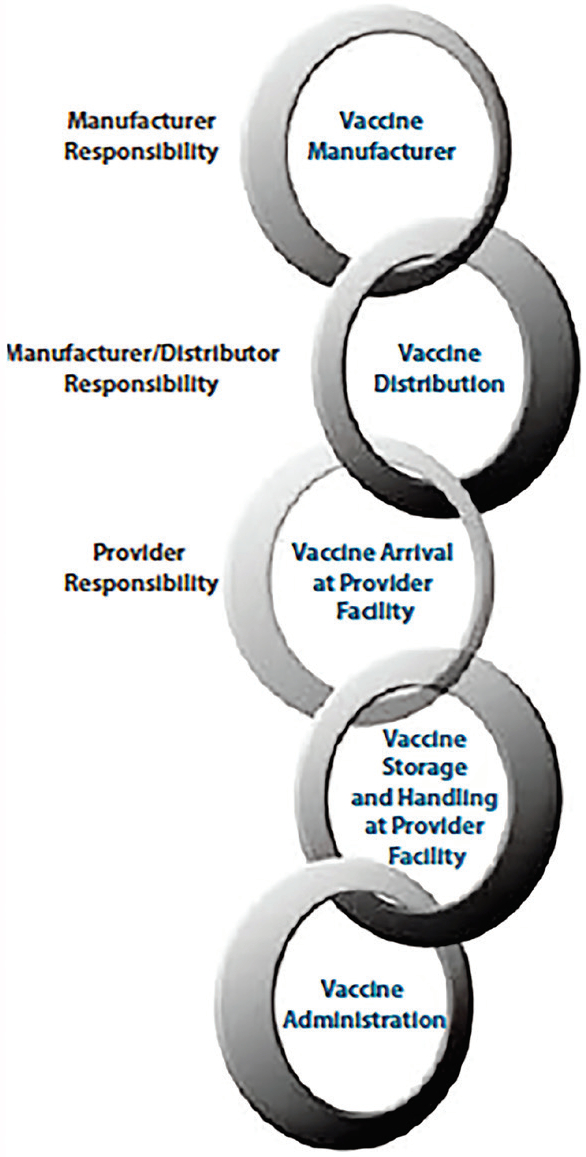 Figure 1 – Cold chain flow chart.
Figure 1 – Cold chain flow chart.High and extreme low temperatures are factors that most often cause degradation. For example, a vaccine can survive higher temperatures for a short period of time and still be deemed safe, but its lifespan may be shortened. Refrigerated vaccines are more susceptible to damage when temperatures dip below freezing. Refrigerators need to be carefully monitored to ensure the samples are kept in appropriate and auditable environmental conditions. The storage environment needs to be temperature-mapped with relevant controls in place to avoid temperature extremes.
Although The Joint Commission, an independent, not-for-profit organization that accredits and certifies nearly 21,000 healthcare organizations and programs in the United States, does not specifically require temperature logs for freezers and refrigerators, it does stipulate that medication be stored according to the manufacturer’s recommendations and that facilities maintain and monitor equipment performance.3 Facilities must track temperatures on a regular basis and note deviations from the required ranges for all stored drugs. A process must be implemented for disposing of medication that no longer meets the recommended temperature ranges.
According to the CDC’s Vaccine Storage and Handling Toolkit,4 thermometers used to monitor vaccine temperature should be:
- Digital if a remote monitoring system with a sensor is not being used
- Equipped with a remote probe and suitable for the temperature range of the application
- Submerged in glycol or glass beads
- Calibrated with a Certificate of Traceability
- Calibrated by an accredited laboratory.
Moreover, thermometers should be accurate to ±1 °F and include an external alarm system with a min/max display and reset button.
Refrigerators and freezers overview
The CDC recommends standalone units that either refrigerate or freeze. All drawers should be removable; units should stabilize temperature with water bottles and frozen coolant packs, and it should not be possible to unplug the units. Full-size and under-the-counter pharmaceutical-grade refrigerators are ideal. Dorm-style or bar-style units are not recommended for primary or temporary storage.
These three simple steps can protect vaccine supplies from temperature-related problems. They should be followed to correctly monitor all aspects of a cooling unit, whether it is an ultralow standard freezer or refrigeration unit. First, identify if the cooling unit is configured with a built-in alarm system typically found in medical- or pharmacological-grade units such as those from Revco, Thermo Fisher Scientific, Aegis Scientific and similar brands. These are ideal for monitoring if the cooling unit has an internal problem such as door left ajar, compressor or fan failure or other internal diagnostic issues. Second, monitor the temperature independently of the built-in system. If the cooling unit fails to trip the alarm output, the monitoring system can still record and monitor temperatures, thus preventing any loss when a cooling unit fails to trip its alarm output. The last precaution and simple measure to take to prevent vaccine loss is to monitor the power at each freezer’s outlet with a power-out sensor. If a breaker trips and power is lost at the cooling unit, monitoring the power will provide ample enough time to take corrective action rather than waiting until the vaccine temperature limits are at a crucial point.
Most medical-grade refrigerators have access ports to allow for third-party temperature probes, which is best practice. However, users can drill a hole in the side, insert the probe and then seal the hole, or simply place the probe in the refrigerator and run the wire through the door opening. The gasket around the door should make a strong enough seal around the wire to keep the outside air from seeping in.
 Figure 2 – Ultralow-temperature sensor (Sensaphone, Aston, Penn.).
Figure 2 – Ultralow-temperature sensor (Sensaphone, Aston, Penn.).Thermometers vs sensors vs probes
There are different methods with which to measure temperature inside a refrigerator or freezer. Thermometers are the most basic. Sensor probes (see Figure 2) detect or measure temperature or other physical properties, and are wired or connected wirelessly to a more intelligent device that continually displays and records the temperature readings. These are typically called data recorders or remote monitoring systems. Probes, which are sensors in rod form, are inserted into the environment being measured and are connected to another device that records the temperature reading. A probe can be used with or without a temperature buffer.
Temperature buffers
 Figure 3 – Temperature buffer.
Figure 3 – Temperature buffer.Buffers act as a cushion against temperature fluctuations in a freezer or refrigerator. A typical buffer (Figure 3) is a bottle filled with glycol solution or glass beads in which a probe or sensor is inserted. Without a buffer, the probe or sensor measures the atmosphere inside the storage unit, which can change rapidly with defrost cycles, door openings or fans circulating the air. The air temperature of the storage unit changes much more quickly than the temperature of vaccines and pharmaceutical products, so a buffered probe reading more accurately represents the temperature of the stored assets.
Continuous vs manual recording of storage temperatures
It is important to keep an ongoing record of temperatures for documentation that items were stored correctly. Data-logging devices have built-in or connected temperature probes and are positioned at the refrigerator or freezer. They continuously measure the temperature in the refrigerator/freezer and store the data locally on the device. With digital data-loggers, users can set alarms to alert when temperatures go out of range and can record events with far greater accuracy than chart readers or thermometers.
A remote monitoring system includes a data-logger and can automatically store data in a remote location that can be accessed by any desktop, tablet or phone. Laboratory personnel can log data at customizable intervals and log separate intervals when in an alarm state. Data is downloaded remotely through a website, either via a local network or cloud, where it can be printed or exported in various formats. Remote monitoring systems also have alarms—when a temperature falls outside a preset range, the system sends a telephone call, text or e-mail to the designated contacts.
More advanced systems allow users to log both the highest and lowest temperatures over a set period of time. This provides a snapshot showing whether the temperatures were within range that day, which is especially convenient when the person responsible for temperatures is not the same as the person designated to receive alarm notifications.
CDC recommendations for data-loggers and remote monitoring systems
- Digital display on the outside of the storage unit for reading temperatures without opening the door
- Detachable
- Probe in a bottle filled with a thermal buffer, like glycol, which more closely reflects vaccine temperatures; vaccine temperatures have been found to be more thermo-stable than air temperature, which fluctuates with defrost cycles and opening and closing of the unit door
- Alert for out-of-range temperatures
- Accuracy to within ±1 °F (±5 °C)
- Low-battery indicator
- Continuous monitoring and recording capabilities to track and record temperatures over time
- Display of current, minimum and maximum temperatures, which indicate the coldest and warmest temperatures recorded since the device was reset.
Calibration
The CDC recommends using calibrated temperature monitoring devices with a Certificate of Traceability and Calibration Testing. It is important that calibration testing is performed by a laboratory accredited by an International Laboratory-Accredited Cooperation (ILAC) Mutual Recognition Arrangement (MRA) signatory body.
An alternative is to purchase monitoring equipment from a manufacturer that provides a Certificate of Calibration. The documentation should show that calibration testing was performed that meets International Organization for Standardization/International Electrotechnical Commission (ISO/IEC) 17025 international standards. Once the data-logger is installed, the CDC recommends calibration testing every one to two years to ensure accuracy.
References
- www.cdc.gov/mmwr/preview/mmwrhtml/mm5242a6.htm
- Shafaat, K.; Hussain, A. et al. An overview: storage of pharmaceutical products. World J. Pharmacy and Pharmaceutical Sci. 2013, 2.5, 2499–2515.
- www.jointcommission.org/standards_information/jcfaqdetails.aspx? StandardsFaqId=1280&ProgramId=46
- www.cdc.gov/vaccines/hcp/admin/storage/toolkit/
Rob Fusco is director of Business Development, Sensaphone, 901 Tryens Rd., Aston, Penn. 19014, U.S.A.; tel.: 877-373-2700; e-mail: [email protected]; www.sensaphone.com