Featured Article
Diverse research and industrial questions demand a range of technologies
A walk along most any body of water—inland or ocean—will soon reveal plastic waste, and this is just the big things, like soda bottles. Pollution levels are even more troublesome when water is analyzed for microplastics, which are pieces smaller than 5 millimeters. A team of scientists from the Switzerland-based University of Basel, Intertek’s Basel laboratory and Canada’s University of Alberta, analyzed the River Rhine1 and found microplastics in every sample, and an average of almost 900,000 particles per square kilometer.
When asked why scientists would analyze water from the Rhine for microplastics, Peter Mühlschlegel, project manager for microscopy and spectroscopy at the Intertek Basel laboratory, said, “Larger plastic parts like abandoned fishing nets or plastic bags catch the interest of the public since they pose an immediate danger to wildlife like seabirds, seals or turtles; however, it is highly likely that microplastics can be found in almost all rivers, lakes and oceans.”
Microplastics come from various sources. “They can result from a breakdown of plastic waste, textile fibers or occur as intermediate products in plastic production or as small pellets that are used in personal-care products,” Mühlschlegel explains. “To understand and assess the impact of microplastics on the different ecosystems, one important task is to study and document their distribution in the environment.”
Microplastics research on the Rhine
Researchers from the Universities of Basel and Alberta collected and processed the samples, which were then analyzed by Intertek’s scientists. “A simple visual and microscopic classification of microplastics is time-consuming and prone to errors and artifacts, especially in the size range below 500 micrometers,” says Mühlschlegel. “Raman and Fourier transform infrared spectroscopy (FTIR) are thus considered a prerequisite for a positive microplastic identification.” A spectral fingerprint of a microplastic sample can be compared with a database to group the samples, Mühlschlegel points out, “in main material classes like polyolefin, polyester or polyvinylchloride.”
The scientists also used other imaging technologies. “Scanning electron microscopy (SEM), in combination with elemental detection by energy-dispersive X-ray spectroscopy (EDX), ideally complements IR and Raman spectroscopy,” Mühlschlegel explains. “While the latter are generally sufficient to group microparticles in main organic polymer classes, analysis by SEM-EDX characterizes inorganic materials by elemental composition and morphology.” Silicates or inorganic fillers are examples of materials that can be determined by SEM-EDX.
Beyond collecting and identifying the microplastics, more work lies ahead. “One obvious objective of further studies is to identify source and origin or former use of the identified microplastic particles, since this could help to reduce their levels in the future,” Mühlschlegel says.
Characterizing with cryo
What is considered a particle varies from one field to another. To analyze tiny biological particles, like proteins or nucleic acids, many scientists use cryo-electron microscopy (cryo-EM).2 Typically, structural biologists use X-ray crystallography. Cryo-EM—a form of transmission EM—keeps samples at cryogenic temperatures, usually with liquid nitrogen, and is not limited to samples that easily crystallize.
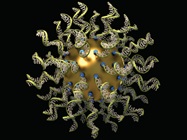
 In one field, a bit of plastic makes up a particle, and in another, it might be a small chunk of biological materials, such as the strands of RNA on this sphere. (Image courtesy of Quintin Anderson, The Seagull Company, and Chad Mirkin and Sarah Petrosko, Northwestern University.)
In one field, a bit of plastic makes up a particle, and in another, it might be a small chunk of biological materials, such as the strands of RNA on this sphere. (Image courtesy of Quintin Anderson, The Seagull Company, and Chad Mirkin and Sarah Petrosko, Northwestern University.)Cryo-EM still needs more fine-tuning for biological particles, which can come apart or jumble into unnatural orientations in preparation for this analysis. One team of scientists developed a technique that keeps the biological sample inside a protective structure of DNA.3 They reported: “[T]his work provides an original approach to exert experimental control over the orientations of individual protein complexes and protect them from harmful forces during cryo-EM sample preparation. As the field of cryo-EM structure determination keeps growing, the physics involved in its sample preparation will become clearer, and new concepts in sample preparation will continue to emerge.”
Cryo-EM could be applied to many processes, including developing new medicines. Scientists from the Max Planck Institute of Molecular Physiology in Dortmund, Germany, wrote: “To understand how proteins function at the atomic level and how mutations lead to dysfunctional proteins, high-resolution structures are needed.”4 They concluded: “Hopefully, cryo-EM will soon extend beyond the reach of structural biology, and become one of the key tools in the toolkit of drug discovery research.”
Cryo-EM can also be used beyond biology. Scientists at Intertek used cryo- EM to study particles in cosmetics, and the results showed some structures smaller than 100 nanometers (download whitepaper at Understanding Micro- and Nano-Formulations: Application of High Pressure Freezing and cryo-Electron Microscopy). Some regulations require special labeling when a product contains such nanosized particles, and making that determination depends on technology that can perform the analysis.
Evolving equipment
 The Litesizer measures particle size and other features. (Image courtesy of Anton Paar.)
The Litesizer measures particle size and other features. (Image courtesy of Anton Paar.)The tools that scientists use in particle analysis keep improving, like the new Litesizer from Anton Paar in Graz, Austria. “Litesizer particle analyzers measure particle size, zeta potential, molecular mass and transmittance by light scattering technology with ingeniously simple software,” says product manager Betty Petrillo. “With the new Litesizer 500, you can determine the particle size and transmittance on a wide variety of samples.” She adds, “It gives you rapid and accurate insight into your particle systems, and provides the tools for optimizing them by revealing how they change with time, pH, temperature and concentration.”
The Litesizer 500 also enables the user to measure zeta potential. Petrillo says, “The unique omega-shaped cuvette for zeta potential combined with patented cmPALS [continuously monitored phase-analysis light scattering] technology guarantees fast, stable and reproducible zeta potential measurements, even on sensitive and turbid samples.” She also notes that “the Litesizer 500 offers a choice of scattering angle, which gives you the optimal measurement conditions whether the sample is concentrated or dilute.”
This technology depends on many key features, and one pointed out by Petrillo is “a very robust optical bench that enables the accurate detection of even low-intensity signals, reduces the effect of vibrations and ensures that measurements remain unaffected by dust or temperature fluctuations.” She adds, “Litesizer particle analyzers provide highly developed measurement algorithms, enabling the resolution of several different particle sizes in a single suspension.”
These devices can be used in many industries, including chemical, environmental analysis, food and beverage, manufacturing and medicine. In the pharmaceutical industry, says Petrillo, the Litesizer is used “in quality control to investigate the presence of possible aggregates in parenteral products which would represent a significant risk for intravenous applications.” Some life scientists use this device to study “how the particle size and zeta potential of microRNA drug delivery systems impact their cellular uptake and distribution when used as a therapeutic strategy to prevent metabolic complications associated with obesity,” Petrillo explains.
Analyzing particles and making use of the results depends on sophisticated devices and algorithms, plus innovative scientists matching the technology with the right questions. That makes particle analysis applicable to microplastics, medicine and much more.
References
- Mani, T.; Hauk, Armin. et al. Microplastics profile along the Rhine river. Sci. Reports 2016; doi: 10.1038/srep17988.
- Callaway, E. The revolution will not be crystallized: a new method sweeps through structural biology. Nature 2015; doi:10.1038/525172a.
- Martin, T.G.; Bharat, T.A.M. et al. Design of a molecular support for cryo-EM structure determination. PNAS 2016; doi:10.1073/ pnas.1612720113.
- Merino, F.; Raunser, S. Cryo-EM as a tool for structure-based drug development. Angewandte Chemie International Edition 2016; doi: 10.1002/anie.201608432.
Mike May is a freelance writer and editor living in Florida. He can be reached at [email protected].