Purified water is the most common reagent in research laboratories. Generally, ultrapure water (Type I) and pure water (Type II) are used in complex applications, while general-grade water (Type III) is used in general laboratory activities.
To establish consistency in grading purified water, organizations such as the American Society for Testing and Materials (ASTM) have established uniform standards of water purity. Based on D1193-06, the ASTM has specified the parameters of four types of water—Types I through IV—with Type I being the purest (see Table 1).
Table 1 – Water quality parameters for ASTM types 
The most common breakdown of the types of water can be seen in Table 2—Types I* through III—with Type I* being the purest.
Table 2 –Water quality parameters for common grades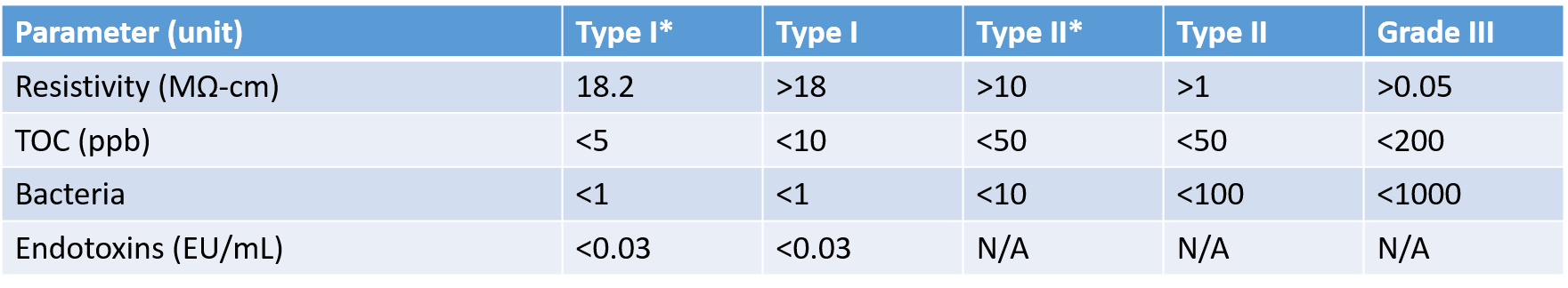
Removal of contaminants
To purify water, contaminants, such as inorganic ions, organic molecules, particulates, colloids, bacteria, pyrogens, and nucleases, must be removed. One or more purification technologies may be used.
Activated carbon
Activated carbon is used in pretreatment cartridges, composite vent filters, and final purification cartridges. It contains a maze of tiny pores with sizes ranging from 500 to 1000 nm and a surface area of about 1000 m2/g. The nature of this surface allows adsorption of organic impurities from the water and catalytic decomposition of free chlorines and chloramines.
Microporous depth filter
This filter provides an entrapment/adsorption barrier for the removal of large suspended particles and some colloids from the water entering the purification process. They are typically rated at 5–10 µm, and, combined with an activated carbon treatment, these filters act to protect subsequent reverse osmosis systems from fouling and blockage.
Reverse osmosis membranes
These membranes are extremely fine filters that reject water contaminants less than 1 nm in diameter. Typically, >90% of ionic impurity, most organic impurity, and nearly all particulates, bacteria, and biomolecules are removed from the filtrate or permeate water.
Ion-exchange resins
These resins are often used as part of a final treatment step. Single-use purification packs typically use a mixture of ion-exchange resins and other media. When used to deionize water, charged impurities are retained on these resins, while H and OH ions are released to replace them. These resins remove ions and produce a water resistivity of up to 18.2 MΩ-cm.
Electrodeionization process
This process combines ion-exchange resins and ion-selective membranes, which are used to move ionic impurities into a waste or concentrate water stream, leaving purified product. As impurities leave via the concentrate water stream, their buildup does not exhaust the resin, and it prolongs the resin lifespan. Water resistivity of >15 MΩ-cm may be achieved by using this process.
Twin-bed ion-exchange process
Deionization processes in which ion-exchange resins may be used to retain impurities have a finite capacity to the impurity. Once that capacity is reached, traces of weakly ionized impurities will begin to elute into the product water, and finally the product resistivity will fall below 18.2 MΩ-cm. A twin-bed ion-exchange process that utilizes a primary purification cartridge in line with a polishing purification cartridge and with inter-stage resistivity monitoring allows for retention of any impurity release during exhaustion of the primary cartridge by the secondary polishing cartridge.
Ultraviolet light
UV light is used to photooxidize organic impurities and/or inactivate microorganisms in water. Photooxidation of organic impurities results in polar or charged species that are subsequently removed by ion-exchange processes. The UV lamp forms part of a polishing treatment loop, including ion exchange, through which water is repeatedly circulated to maintain quality. Water with total organic carbon of <5 ppbc and bacteria at < 1CFU/mL may be achieved.
Submicron filtration
Submicron filtration with micro-, ultramicro-, and ultrafilters (1–200 nm) are used as part of a polishing look or at the point of use. Fine filtration is applied to remove bacteria (live or dead) and biologically active molecules. These filters have pores smaller than their intended target and retain the impurity while allowing water to pass through. Impurities that can be removed by submicron filtration include bacteria, colloids, enzymes, endotoxins, and particulates.
Purification strategies
In selecting a purification system, a key consideration is the type of application and corresponding requirement for the level of water purity. For example, Type I* water, which has a resistivity of 18.2 MΩ-cm, is required for critical applications, such as ICP/MS (inductively coupled/plasma mass spectrometry); Type I water can be used for applications such as HPLC; Type II* and II water can be used for histology and spectrophotometry; Type III water is recommended for noncritical applications, such as autoclave feeds. Typical uses for each type of water are shown in Table 3.
Table 3 – Typical uses of purified water
There are several options available to produce purified water. Key considerations are the type of research application, feedwater source (prepurified or tap water), daily volume of water to be processed, need for water storage, and continuous monitoring of water quality by TOC monitoring or resistivity measurement.
Point-of-use filters
Traditionally, point-of-use filters have been used to purify water at a single water source. While point-of-use purified water may be distributed to more than one outlet, one drawback is the low volume production of these filters, which makes them best suited for light use applications.
Integrated purification systems
Integrated purification systems enable a total system approach, combining technologies such as reverse osmosis, ion exchange, UV, and micro- or ultrafiltration. The major benefit of integrated purification systems over point-of-use filters is that the levels of bacteria or endotoxins may be further filtered or recycled before use.
The type of application and level of water purity dictate the configuration of the integrated system. For example, a basic purification system may include a primary purification pack, followed by a UV lamp and a polishing purification pack to produce Type I water (see Figure 1).
 Figure 1 – Image courtesy of ELGA LabWater.
Figure 1 – Image courtesy of ELGA LabWater.PureSure® technology can also be incorporated into the water purification system, which consists of two purification packs. This ensures the removal of weakly ionized impurities and gives advanced warning of purification pack exhaustion without compromising final water purity (see Figure 2).
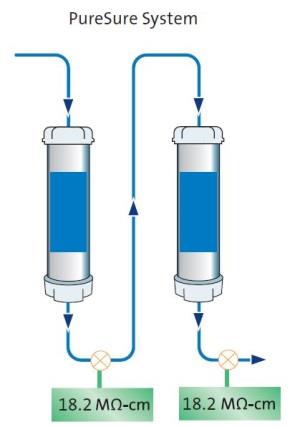 Figure 2 – Image courtesy of ELGA LabWater.
Figure 2 – Image courtesy of ELGA LabWater.A more comprehensive integrated system may include an ultramicrofiltration system following the primary purification pack, UV lamp, and polishing purification pack (see Figure 3).
Optionally, a point-of-use filter following the purification pack will prevent back-contamination and provide a final filter directly before delivery.
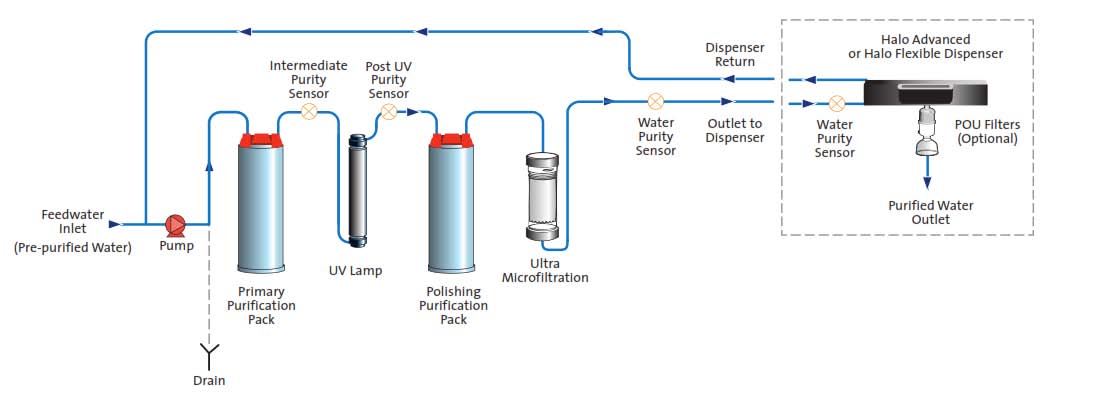 Figure 3 – Image courtesy of ELGA LabWater.
Figure 3 – Image courtesy of ELGA LabWater.With tap water as the source, the configuration of a purification system may include reverse osmosis ahead of the purification pack (see Figure 4).
 Figure 4 – Image courtesy of ELGA LabWater.
Figure 4 – Image courtesy of ELGA LabWater.Conclusion
There are as many possible configurations of systems for water purification as there are applications and separation challenges. For more information, visit http://www.elgalabwater.com.
Lina Genovesi, Ph.D., JD, is a technical, regulatory, and business writer based in Princeton, NJ, U.S.A.; e-mail: [email protected]; www.linagenovesi.com