Featured Article

High-performance liquid chromatography, also known as high-pressure liquid chromatography (both referred to as HPLC), is a highly versatile, sensitive technique for conducting separations and analyses. In the early twentieth century, a rudimentary “chromatographic” separation technique was employed by Russian botanist Mikhail Tsvet as a method to separate pigments, lending the term “chromatography,” as in chroma (Greek for “color”), to the analytical method. The mathematical formula for quantifying the behavior of phase separations in the HPLC method was presented by Martin and Synge in the 1940s. They theorized that high pressure is required to move a mobile phase through a stationary phase. In 1969, Waters Corporation launched the ALC100, the first commercial HPLC system. Dionex Corporation released the first commercialized ion exchange columns in 1971.
The retention time of an HPLC sample is defined as the amount of time between injection and elution of the sample from a column. The retention factors of two compounds being separated via HPLC must differ, since column selectivity is based on the ratio of the retention factors. Therefore, if two compounds share the same retention factor, they elute simultaneously.
Various options are available that allow users to configure optimized systems from a range of modular components. Alternatively, vendors offer an array of turnkey systems for specific separations, as well as a diverse selection of columns that integrate with existing equipment. This article provides an overview of HPLC methodology and instrumentation, as well as purchasing considerations for users who are new to HPLC.
HPLC basics
From sample injection to detection, a basic HPLC system comprises the following: a solvent reservoir (if equipped), solvent pump, injector system, column, detector, data processor (a computer) and some form of data output (a printer or digital file type).
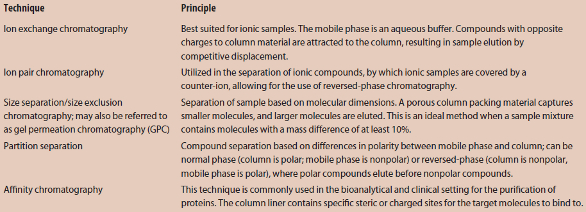
The workhorse of the HPLC system is the column. Typically, an HPLC column is a stainless steel tube with compression fittings at both ends. Separation of compounds based on molecular polarity (e.g., separating immiscible liquids) is a common analytical technique. Additionally, HPLC separations can be performed on the basis of molecular charge, affinity, shape and size, as well as molecular isomerism (refer to Table 1 for an introduction to different HPLC separation techniques). Changing the polarity between the mobile phase (sample and solvent) and the column packing material (a solid liner) changes the nature of the separation.
Table 1 – Common HPLC techniques
HPLC analyses
To perform an HPLC separation, the user injects a sample dissolved in a solvent into a packed column. The column is lined with a solid separating material. As the solvent–sample matrix is injected into the column and passes through it, the sample molecules are effectively washed out and separated by molecular affinity. Therefore, compounds with the highest affinity for a certain column packing material wash out last. The elution of separated compounds is analyzed by an HPLC detector that processes the behavior into rising and falling peaks. Among the many commercially available detectors, one of the most commonly used is the variable ultraviolet (UV) absorption detector.
Purchasing considerations for HPLC systems
In choosing an HPLC system, the primary considerations are the environment in which the instrument will be used, hardware demands, compatibility with existing equipment, requirements for time savings and budget. A fundamental understanding of the targeted molecular interactions among analytes, solvents and column packing materials in separations is necessary for selecting the appropriate chromatography technique (Table 1). It is wise to consider the terms of support offered by the manufacturer; although high pressure brings high performance, it does so at the risk of leaks, sample loss and other malfunctions during the equipment’s operating life.
HPLC systems can be separated into four types, distinguished by their application, complexity and price, in ascending order (Table 2).
Table 2 – Sorting HPLC instruments by type and application

Choosing an HPLC instrument
Entry-level, accessible HPLC instruments are now more advanced than ever. For example, the Breeze 2 HPLC from Waters is preconfigured for different analyses. Out of the box, it comes with a binary or isocratic pump, an automated or manual injector and UV/VIS, refractive index, multi-λ fluorescence, evaporative light scattering or photodiode array allows users to choose between five different solvents, different purification techniques and interchangeable pump heads.
 Figure 1 – The Prominence nano HPLC from Shimadzu Scientific Instruments permits configuration of ion exchange and reversed-phase chromatography methods.
Figure 1 – The Prominence nano HPLC from Shimadzu Scientific Instruments permits configuration of ion exchange and reversed-phase chromatography methods.Moving onto semipreparative systems with analytical capabilities, the benchtop-friendly BLC-10 fixed wavelength isocratic HPLC system from Buck Scientific features changeable flow cells that can be changed from preparative (2 mm pathlength/4 mL) to analytical mode (7 mm pathlength/10 mL). An affordable, automated, research-grade analytical HPLC is the Merit Automatic Binary Gradient and Scanning HPLC system from Cecil Instruments Ltd., which is built to offer hands-free operation with highly sensitive UV/VIS detection.
An advanced, efficient, combined mode system such as the Prominence nano HPLC from Shimadzu Scientific Instruments (Figure 1) allows for the configuration of two-dimensional chromatography methods (ion exchange and reversed-phase). Combining these methods is excellent for complicated analyses, and the Prominence nano in 2-D mode can serve as an online proteome analyzer when used with a nano ESI (electrospray ionization) interfaceequipped LC/MS or a MALDI (matrix-assisted laser desorption ionization) spotter.
A list of HPLC system manufacturers is given in Table 3.
Table 3 – Manufacturers of HPLC systems

Emilia Raszkiewicz is managing editor, American Laboratory; [email protected]