Featured Article

Independent Cannabis laboratories provide quality assurance to the medical and recreational Cannabis market in the same manner that independent quality assurance labs nationwide help to guarantee the safety of foods, medicines and manufactured goods. Cannabis testing labs receive samples from commercial growers, producers of concentrated extracts (oils) and makers of infused products. Depending on the state requirements for compliance and customer interest, these products are assayed for microbiological contaminants, cannabinoid concentrations, pesticide residues, residual solvents and/or terpenes. Although some of the analytes are common among QA labs, a number—such as the cannabinoids—are uniquely associated with Cannabis. Methods used for the analysis of cannabinoids are often developed and validated by the individual labs. More common analytes, such as fungi, bacteria and pesticide residues, are often assayed using existing techniques, with some modifications to account for the unique matrix effects and desired list of target compounds.
Standard methods offer an ideal solution for many laboratories by providing a route to expedited method development. By definition, standard methods have undergone validation studies that compare the results between multiple labs, instruments and analysts. Because of the inherent rigor and peer review associated with this process, these methods provide a turn-key solution that allows laboratories to confidently test for analytes with an abridged validation process. These methods— offered by organizations such as the Food and Drug Administration (FDA), Environmental Protection Agency (EPA), Association of Official Analytical Chemists (AOAC), American Society for Testing and Materials (ASTM) and many others—allow consumers to easily compare labs and impart a degree of confidence in testing. This is a benefit that has thus far been unavailable to operators of Cannabis laboratories.
Challenges to Cannabis analysis
Due to federal restrictions, extracts and materials containing the active ingredient Δ9-tetrahydrocannibinol (d9-THC) or other cannabinoids are not permitted for transport without a Drug Enforcement Agency (DEA) exemption. This prohibition impacts laboratories attempting any kind of nationwide proficiency testing (PT) as well as national interlab comparisons for the development of standard methods. Proficiency testing can be conducted, but never in the same matrix as the samples received by Cannabis labs. Providers of these PTs must therefore offer the samples either in a premade solution or in some matrix determined to be similar to the Cannabis flower. The American Oil Chemists’ Society (AOCS) and Emerald Scientific (San Luis Obispo, Calif.) have developed a PT for many of the analytes assayed by Cannabis labs, but they are not offered in the correct matrix. While invaluable for assessing the calibrations in use in Cannabis labs, the test does not assess sample preparation. Certified reference material (CRM) producers offering a DEA-exempt standard are only able to offer materials at concentrations of 1.0 mg/mL or less, limiting their use in spike recovery.1 Despite the fact that over 100 cannabinoids have been identified in Cannabis, fewer than 20 of these compounds are available commercially.
Cannabis offers a unique challenge in regard to contaminant testing due to inapplicable target lists. For example, USP <467> describes testing for residual solvents on pharmaceuticals and establishes maximum residue limits (MRLs) for each solvent. Low-molecular-weight hydrocarbons (LMW HCs) are not included in this list (such as propane, n-butane and isobutane) and therefore default to a 5000-ppm limit according to this document. Although all of these compounds enjoy a G.R.A.S. (Generally Regarded as Safe) status with the FDA, these hydrocarbons are routinely used in the manufacture of concentrated Cannabis oils and their residues are of particular interest to the industry. Regulations designed specifically for the Cannabis industry2 have identified this, and many have created MRLs for these LMW HCs while taking limits on other residues directly from USP <467>.
Multiresidue methods for pesticide residues are readily available, but rarely do they include all residues of concern to the Cannabis industry. Modifications are thus usually made to existing methods and are validated by each laboratory. This lack of uniformity in the methods utilized is often confusing to the consumer, and leads to highly varied target lists from the different labs. Limits of detection and quantification are also typically different, leading to situations in which the same material may pass a test in one lab but fail in another. There is no analogous agricultural commodity with established pesticide MRLs in line with Cannabis. Tobacco may seem like an obvious choice, but the FDA and EPA have declined to establish MRLs for domestically grown tobacco.3 Lacking this information, regulators and Cannabis lab operators are left to determine what constitutes a safe residue level. Some states, such as Oregon, have taken a proactive approach, creating a clearly defined target list that includes most of the analytes in common use during Cannabis cultivation. In some cases the language is vague, stating that the material should be tested for chemical contaminants without providing an MRL or target list.
Cannabis labs are routinely confronted with clients that have a poor understanding of variation within natural products. Because most states do not have a provision for laboratories to perform sampling themselves, it is up to the client to collect the samples and provide them to the lab. Due to the high monetary value of the material, these samples are often the bare minimum needed to conduct the test, leading to predictably wide variability between sample submissions. When combined with the use of different methods and instrumentation, there is very little baseline for understanding whether the source of the bias is from the laboratories or the sampling.
Efforts to date
Since 2011, the Association of Commercial Cannabis Laboratories (ACCL) has been advocating for its members and greater unity in methodologies.4 Regular proficiency testing, managed by Emerald Scientific and the AOCS, provides an additional measure of credibility to members. Efforts are underway to develop standard methods through ACCL and AOAC, and members are typically willing to share extraction techniques and calibration methodologies.
Table 1 – Changes in availability of certified reference materials from 2009 to 2016
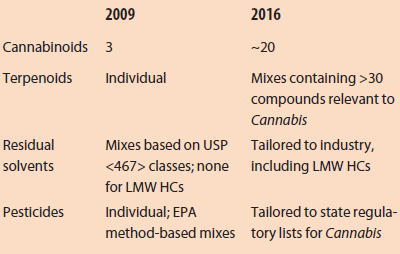
Since the first laboratories began operating in 2007, the provisioning of certified reference standards that are tailored to the Cannabis industry has progressed substantially (Table 1). The growing availability of individual and mixed standards has proceeded in step with the technological improvements of the lab industry and increasing specificity in state regulations. As the number of labs and the desire for QA testing has become routine, the number of providers fulfilling this niche has increased.
Cannabis labs are more accurate and robust in their capabilities than ever before, as demonstrated by proficiency test results. The impression in the industry that labs are highly variable in their results stems directly from the aforementioned reasons, but the fault is often placed on the laboratory. Much of this comes from lack of client fluency in reading and understanding technical data and the inherent variability in analytical results. It is incumbent upon all labs to educate their clientele about natural variability without pushing the blame onto a competitor. Cooperation between competing labs can only lead to improved precision and better agreement between different methods.
Cannabis labs have spent years optimizing their methods for a wide variety of matrices without the benefit of tailored reference standards, application notes or white papers. This is now changing, as public acceptance of Cannabis is growing, and more equipment manufacturers and trade groups are seeing opportunity in this bourgeoning industry. These resources, produced by companies such as Restek, Cerilliant, Sciex, Shimadzu and others, have provided the first steps in achieving greater credibility in the industry. Cannabis laboratories and trade groups such as the ACCL are doing their part by attaining ISO 17025 accreditation and adhering to good laboratory practice in an effort to improve the image that has been unfairly applied to them. As the Cannabis industry matures, labs can be expected to more closely resemble their counterparts in the environmental testing industry.
References
- www.dea.gov/druginfo/ds.shtml
- Rough, L. (2016, March 4). Leafly’s State-by-State Guide to Medical Cannabis Testing Regulations. Retrieved from www.leafly.com: https://www.leafly.com/news/industry/leaflys-state-by-state-guide-to-cannabis-testing-regulations/
- Daley, P.; Lampach, D. et al. Testing Cannabis for contaminants. BOTEC Analysis Corp., Sept 2013.
- www.cacannabislabs.com
David Egerton is vice president of technical operations for CW Analytical, 851 81st Ave., Ste. D, Oakland, Calif. 94621, U.S.A.; tel.: 510-306-4716; e-mail: [email protected]; www.cwanalytical.com