Featured Article
High-throughput screening of new anti-cancer drug candidates has typically been achieved by testing drugs on in vitro two-dimensional monolayer cell cultures grown on flat, rigid surfaces such as plastic. However, these cell culture models do not accurately mimic the three-dimensional in vivo interactions between cancer cells and extracellular matrix (ECM)/stromal cells. Modeling these interactions is important in the discovery of drugs for breast cancer because stromal cells are known to play a role in tumorigenesis,1 including promoting breast cancer growth and metastasis,2,3 making them appealing targets for treatment.
Testing anti-cancer drugs on 3-D co-cultures could provide more reliable results and better predict in vivo responses, and help to avoid unnecessary downstream animal testing and clinical trials.4 Methods to generate 3-D co-cultures are still being optimized, with new technologies showing promise for producing biologically representative models of breast cancer that will likely reduce costs and aid in the discovery of new drugs.
Two-dimensional versus three-dimensional cell cultures
Cells grown in 2-D cultures are usually flatter and more stretched out than they would appear in vivo. As a result, the cells do not behave in the same way as they would in the body because the culture does not adequately mimic the in vivo microenvironment.5 In 3-D culture systems usually employed in cancer studies, cancer cell lines are grown to form aggregates or spheroids either on prefabricated scaffolds or without scaffolds. Within these 3-D architectural structures are increased cell–cell interactions, cell–ECM interactions, and signaling pathway activation, better mimicking the tumor microenvironment found in vivo.6 Three-dimensional cultures have shown greater resistance to anti-cancer drug treatments than 2-D cultures, which has been found to be more similar to what occurs in tumors in vivo.7 In 3-D assays of breast cancer cell lines, cell functionality and morphological differentiation can be largely restored to what is seen in vivo,8 and the heterogeneous cell population is similar to that of tumors by having a mixture of proliferating, hypoxic, and necrotic cells.4
A key advantage of biomimetic 3-D cell cultures over 2-D cultures is that they provide the opportunity to co-culture cancer cells with ECM to mimic in vivo interactions between ECM and cancer cells. This can be an advanced way of modeling the role of ECM/stromal cells in cancer progression and treatment response, which has been overlooked to some extent.
Three-dimensional multicellular cultures as models of cancer
Progress has been made in generating 3-D co-cultures to study the effects of ECM/stromal cell activity on cancer. One study found that primary human adult lung cancer-associated fibroblasts (LuCAFs) may alter human bronchial epithelial cells (HBECs) by modifying biochemical signals conveyed through the ECM and altering the expression of six genes associated with immune responses, apoptosis, mitosis, cell survival, and differentiation.9
While 3-D cell culture shows promise for modeling cancer, some methods have limitations, which may restrict their use in anti-cancer drug discovery.10 For example, 3-D methods that involve the development of a self-assembled spheroid without a surrounding ECM are not ideal as it becomes difficult to study cell–ECM interactions. In addition, some models produce spheroids that vary greatly in size, resulting in high variability within the same flask,11 and it has been suggested that variability in biologically derived matrices may cause nonreproducible results.12 Another drawback of some 3-D models for studying ECM–tumor interactions is that they can lack vasculature, which plays a vital role in tumor growth and survival, as well as drug delivery.13
Nonetheless, much progress is being made to advance the methods used to generate 3-D ECM–tumor models and overcome these limitations. The RAFT 3D Cell Culture System (Lonza, Walkersville, MD) (Figure 1) can produce a vascularized 3-D in vitro ECM–colorectal cancer model that mimics in vivo tumor progression through angiogenic processes.14 A recent study found that intratumor heterogeneity increased cancer invasion into the stroma, demonstrating the system’s potential to produce biomimetic in vitro models to study the microenvironment and its role in tumor progression.
 Figure 1 – RAFT 3D Cell Culture System. (Copyright © Lonza, Basel, Switzerland.)
Figure 1 – RAFT 3D Cell Culture System. (Copyright © Lonza, Basel, Switzerland.)Other researchers explored how natural killer (NK) cells interact with cancer cells and the ECM to provide an anti-cancer effect, which could help to advance cancer immunotherapies.15 The RAFT System was used to produce 3-D colorectal cancer tumoroids within an ECM matrix to which NK cells were added. Induced necrosis and apoptosis of the tumoroids were subsequently monitored. The results showed that compared to a 2-D monolayer of the cancer cells, the 3-D cell model was far superior as an NK cell-mediated cytotoxicity assay; NK cell-mediated cytotoxicity progressed kinetically in a manner consistent with programmed cell death, and cells stayed viable for a much longer period.
It is also possible to produce a biologically representative model of breast cancer by mimicking in vivo heterotypic interactions between human mammary fibroblasts and human mammary epithelial cells in vitro, an important step in exploring the role of these interactions in in vivo breast cancer behavior and treatment.
Case study: 3-D co-culture breast cancer model
A recent study investigated whether a 3-D cell culture system could successfully co-culture human mammary fibroblasts (HMFs, isolated in-house) and human breast cancer epithelial cells (MCF7s, ATCC, Manassas, VA) as a 3-D model of breast cancer. This cancer co-culture, along with a “noncancer” control co-culture made up of HMFs and normal primary human mammary epithelial cells (nHMECs, Lonza), was attempted in both the RAFT System and on a 2-D plastic surface. Cell proliferation and response to an anti-cancer drug, PIK-75 (Selleckchem, Houston, TX), in the cancer and noncancer RAFT co-cultures were measured.
The first step was to construct the 3-D coculture of the nHMEC/MCF7 and HMF cells.
The RAFT System is made up of a collagen matrix, with most of the liquid extracted using specialized absorbers to create physiologically relevant collagen density. A formulation combining fibroblast medium FGM-2 BulletKit and nHMEC medium MEGM BulletKit (both from Lonza), was optimized to grow both cell types in the co-culture. The cells of interest can be embedded in this matrix by either premixing them in the collagen hydrogel prior to application of the absorber or by seeding and growing them on top of the collagen matrix post application (Figure 2). After this, immunocytochemistry imaging was used to assess the morphology of the cells co-cultured using the RAFT System and on a separate 2-D plastic surface.
 Figure 2 – Construction of the 3-D RAFT breast cancer model. (Copyright © Lonza, Basel, Switzerland.)
Figure 2 – Construction of the 3-D RAFT breast cancer model. (Copyright © Lonza, Basel, Switzerland.)The final step involved assessment of cell proliferation and PIK-75 drug efficacy in all the co-cultures and the single cell cultures of the epithelial cells. This was done using the Vialight Plus Cell Proliferation and Cytotoxicity BioAssay (Lonza), which measures cell viability with bioluminescent detection of cellular adenosine triphosphate (ATP). PIK-75 (1 µM) was introduced into the culture medium on day 3, and the cultures were treated with the same drug for five days until day 8 postseeding. A control “no-drug” culture fed with medium spiked with the vehicle (DMSO) was grown in parallel. The Vialight bioassay was performed on days 1, 3, 4, 6, and 8.
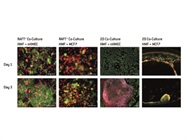
Immunocytochemistry showed that after three days in the 3-D RAFT co-culture, the HMFs had fully spread and stretched out in the collagen matrix, and the epithelial cells (nHMECs and MCF7s) had multiplied on top of it in a 3-D structure to mimic the heterotypic interactions of both cell types in vivo (Figure 3). The morphology of the co-cultured cells was very different from that of cells on the 2-D plastic surface. In the HMF-nHMEC 2D co-culture, the HMFs at high density peeled off the plate or retracted to separate from the nHMECs; in the HMF-MCF7 2D co-culture, the HMFs retracted toward the edge of the plate and aggregated with the MCF7 cells (Figure 3).
 Figure 3 – Immunocytochemistry images of the mammary fibroblast and epithelial cell co-cultures in the RAFT System, in comparison to cells on the 2-D plastic surface. (Copyright © Lonza, Basel, Switzerland.)
Figure 3 – Immunocytochemistry images of the mammary fibroblast and epithelial cell co-cultures in the RAFT System, in comparison to cells on the 2-D plastic surface. (Copyright © Lonza, Basel, Switzerland.)Vialight Bioassay data showed that in the single cell cultures, nHMECs proliferated much more rapidly than MCF7 cells on both surfaces. The nHMECs also proliferated and responded to the drug faster on the 2-D plastic surface than on the RAFT matrix, while MCF7s behaved similarly on both surfaces. In the RAFT System, HMFs stayed viable in the collagen matrix but hardly multiplied (about 20% increase in Vialight read out after seven days). Embedded HMFs did not appear to have any significant effect on the proliferation of MCF7s, or on their response to PIK-75, in the co-culture.
Conclusion
Human mammary epithelial cells and human mammary fibroblasts can be successfully grown in a 3-D co-culture system, using a blend of MEGM BulletKit and FGM-2 BulletKit, to mimic the heterotypic interactions of breast cancer cells and stromal cells in vivo. Immunocytochemistry imaging was shown to be applicable in the RAFT co-culture to reveal the morphology of the cells cultured in three dimensions.
The RAFT System can be used to produce a biologically representative 3-D breast cancer model with stromal cells at a more physiologically relevant density, which is not possible using 2-D cell culture. Other types of fibroblasts (such as cancer-associated fibroblasts) or stromal cells (such as adipose tissue-derived mesenchymal stem cells) could potentially be used to further analyze responses to anti-cancer drugs and explore the role of stromal cells in breast cancer.
References
- Wiseman, B. and Werb, Z. Science2002, 296, 1046–9.
- Karnoub, A.; Dash, A.; et al. Nature2007, 449, 557–63.
- Sasser, A.; Mundy, B. et al. Canc. Lett. 2007, 254, 255–64.
- Kim, J. Semin. Cancer Biol.2005, 15, 365–77.
- Huh, D.; Hamilton, G. et al. Trends Cell Biol.2011, 21, 745–54.
- Nyga, A.; Cheema, U. et al. J. Cell Commun. Signal2011, 5, 239.
- Shield, K.; Ackland, M. et al. Gynecol. Oncol.2009, 113, 143–8.
- Kenny, P.; Lee, G. et al. Mol. Oncol.2007, 1, 84–96.
- Pageau, S.; Sazonova, O. et al. Biomaterials2011, 32, 7169–80.
- Edmondson, R.; Jenkins, B. et al. Assay Drug Dev. Technol.2014, 12, 207–18.
- Gurski, L.; Petrelli, N. et al. Oncol. Issues2010, 25, 20–5.
- Tibbitt, M. and Anseth, K. Biotechnol. Bioeng. 2009, 103, 655–63.
- Yamada, K. and Cukierman, E. Cell2007, 130, 601–10.
- Magdeldin, T.; Lopez-Davila, V. et al. Sci. Rep. 2017, 7, 44–45.
- Larson, B.; Hussain, L. et al. https://www.biotek.com/assets/tech_ resources/3D Natural Killer Cell Mediated Cytotoxicity Assay App Note.pdf
Ying Nie is senior scientist; Krista Garner is research associate; and Lubna Hussain is senior product manager for primary cells, media products, and 3-D culture systems, Lonza, Walkersville, Inc., 8830 Biggs Ford Rd., Walkersville, MD 21793, U.S.A.; tel.: 301-898-7025; e-mail: [email protected]; www. lonza.com.