Featured Article
Historically, HPLC has been the most common analytical method for quantitation of residual active pharmaceutical ingredients (APIs) for cleaning validation (CV) of pharmaceutical production equipment. Over time, manufacturers, regulatory agencies, and industrial groups have changed focus from HPLC to total organic carbon (TOC) analysis as the preferred analytical method for use in cleaning validation. The reasons for this shift include, but are not limited to, better cleaning process understanding for the life cycle of the equipment, decreased cost, increased productivity, and therefore increased profitability. The question remains for those manufacturers that have qualified their cleaning process using HPLC: What are the best practices when changing from HPLC to TOC analysis for cleaning validation?
For manufacturers with validated HPLC methods for cleaning validation, the first step when considering TOC for CV analysis is to examine the feasibility of TOC in lieu of HPLC. The following are three main factors to consider when examining the feasibility of TOC analysis for CV:
- Cleaning process/sample compatibility
- Cleaning limit acceptance criteria
- Product recovery/solubility
Cleaning process/sample compatibility
With regard to cleaning process/sample compatibility, TOC analysis requires aqueous solutions of samples for quantitation of TOC. Organic solvents such as methanol, ethanol, and isopropanol are not suitable solvents to measure TOC. If the existing cleaning cycle has an aqueous solution without any organic solvents in the final rinse, then TOC analysis may be a feasible method. If the existing cleaning process does use organic solvents, can that process be altered to use a final aqueous rinse?
Cleaning limit acceptance criteria
When setting TOC limit acceptance criteria for CV, a worst-case scenario is assumed. This means the most toxic substance/API, with the lowest maximum allowable carryover (MAC) limit from the previous batch of product, is assumed to contribute all the TOC measured in the cleaning sample. Inherently, this substance must have some carbon content in the chemical formula to be suitable for TOC analysis. Based on the MAC limit of that API, conversion from a product limit to a TOC limit can be determined using the carbon content in the chemical formula. The newly determined TOC MAC limit must fall within the linear dynamic range of the TOC instrument to be a feasible method for CV.
Product recovery/solubility
One common misconception regarding solubility of APIs for CV with TOC analysis is that solubility is the limiting factor. Traditionally, insoluble or difficult-to-solubilize compounds can be oxidized and soluble at low concentrations or, when necessary, with pretreatment of the solutions using temperature, agitation, chemistry, and time (TACT). For example, studies have proven that traditionally insoluble or difficult-to-solubilize compounds such as ibuprofen, azithromycin, starch, and lidocaine can all be recovered with excellent linearity by TOC analysis using little to no pretreatment of the sample.
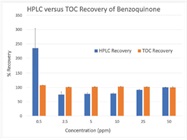
Once feasibility has been determined, a recovery study must be made to show equivalency of recovery and linearity between HPLC and TOC analysis. This work requires preparation of the lowest MAC limit API stock solutions with concentrations bracketed around previously determined API TOC limits. For example, if the API with the lowest MAC limit is benzoquinone (C6H4O2 – 108.09 g/mol) with a product limit of 10 ppm, the TOC limit is calculated to be 6.67 ppm, considering benzoquinone is 66.7% carbon, based on molecular weight. Knowing this limit, the recovery study would want to challenge the HPLC and the TOC recovery and linearity above and below the 6.67 ppm limit.

In this example, the recovery and linearity of the HPLC and the TOC instruments are challenged one order of magnitude above and below the TOC MAC limit. Results for both the HPLC and TOC samples are shown in Figure 1. As can be seen, not only does the TOC instrument have superior linearity to the HPLC analyzing the same samples, it also has more accurate recovery. With the recovery and linearity data available for analysis, a definitive determination can be made about the equivalency of the two analytical methods for this specific API. If the recovery or linearity did not pass the acceptance criteria, sample pretreatment using TACT may be necessary.
 Figure 1 – HPLC versus TOC instrument response to benzoquinone.
Figure 1 – HPLC versus TOC instrument response to benzoquinone.Following the recovery study, the next step is to run a bridge study of actual cleanout samples on the HPLC and TOC instruments side by side. Cleanout samples, whether they be rinsate or swab, need to be run in parallel with the HPLC and TOC instruments. The expectation is that the TOC value is equal to or greater than the HPLC value due to the known additional contributions to TOC from detergents, excipients, fillers, etc. If the cleanout samples pass both HPLC and TOC, no additional work is required. If the cleanout samples fail both HPLC and TOC, the cleaning process must be evaluated. What happens, though, if the cleanout samples pass HPLC but fail TOC? What is the best practice guidance in that scenario?
In a scenario in which the HPLC results pass but the TOC results fail acceptance criteria, the value of process understanding is highlighted using TOC analysis. The use of HPLC quantitation of API to release equipment to production may result in carryover of residual soils from other sources. As a product-specific method, HPLC may not measure these soils. These soils can have detrimental consequences to products affecting yield, efficacy, or even consumer safety. Data from TOC analysis can demonstrate that equipment is still dirty, thus prompting revisions to the cleaning process, again using TACT as guidelines to reduce residual soils.
Once the feasibility, recovery, and bridge studies have all demonstrated the efficacy of TOC analysis for CV, internal standard operating procedures can be changed to reflect the new work flow for releasing equipment for production. Figure 2 summarizes the steps and best practices when changing from HPLC to TOC for cleaning validation. Benefits to this new analytical method include reduced sample analysis time, reduced consumable costs, and increased productivity.
 Figure 2 – Steps and best practices when changing from HPLC to TOC analysis for cleaning validation.
Figure 2 – Steps and best practices when changing from HPLC to TOC analysis for cleaning validation.Lukas Swanson, M. Eng., is life science applications engineer, SUEZ (formerly GE Analytical Instruments), 6060 Spine Rd., Boulder, CO 80301, U.S.A.; e-mail: [email protected]; www.sieversinstruments.com