Featured Article
Rigorous temperature control of biological samples is crucial to maintain their optimal viability and function. It is therefore imperative to safeguard a continuous, tightly controlled cold chain-of-custody. Expert cold-chain logistics, seamless chain-of-custody management, and precise control over sample inventory are necessary to avoid inadvertent loss of valuable materials during transport. This is especially important when moving entire laboratories. This article highlights technologies that help ensure optimal maintenance of the cold chain, and therefore sample integrity, when relocating valuable temperature-sensitive materials.
Challenges during transport of temperature-sensitive biomaterial
Most biological materials are extremely temperature-sensitive and must be stored at low temperatures, often in a frozen state. For bacterial, viral, or eukaryotic cell suspensions, exact and reliable maintenance of the appropriate storage temperature is crucial to maintain optimal post-thaw cell viability. Therefore, strict adherence to cold-chain requirements for biosamples is essential for any laboratory or biobank. Eukaryotic cell suspensions, in particular, require storage at temperatures that are below the threshold for the glass transition phase of water Tg-H2O, approximately –135 °C, to maximize viscosity and minimize biological activity. This article focuses on cold-chain requirements for this specific biological material.
Adherence to these cold-chain requirements can be challenging, particularly when the biosamples have to be transported, either from a manufacturing or storage site to a point-of-use or between research centers. Cold-chain logistics are also indispensable when an entire laboratory relocates its operations, or in emergency situations. Proper temperature management during transport of such biological materials can be difficult. Carriage between cities (or countries) can proceed for days. Even short transport processes, such as the relocation of samples between laboratories within the same building, pose temperature stability challenges. Luckily, technological solutions exist that specifically address these challenges. How precise, and how quickly, do temperature maintenance procedures have to take effect to avoid sample deterioration?
Effect of temperature fluctuations on sample integrity and viability
The importance of strict temperature control for biological materials is often underestimated. In everyday operations in a standard research laboratory, vials that contain valuable cell cultures may frequently experience transient warming events (TWEs). These events may occur when a researcher accesses storage boxes in freezers or when specific vials in a storage box are needed for further experimentation. TWEs are of considerable duration when the exact location of the desired vials is difficult to identify, resulting in a prolonged stay of the desired biosamples, and of samples that are stored adjacent from the desired ones, outside their appropriate environment.
To pinpoint the time of exposure to ambient temperatures after which biological samples may begin to experience detrimental warming, recent experiments investigated the changes in temperatures of a sample when it was removed from a –190 °C vapor-phased liquid nitrogen (LN2) freezer.1 Several extraction times were investigated in an attempt to replicate various operating protocols of different labs. The upshot was that under the conditions tested, a 1-mL sample in an empty storage box may reach Tg-H2O (–135 °C) after 135 seconds of exposure to ambient temperatures (Figure 1). Similar experiments determined that the rate of warming for samples that are moved from –190 °C to room temperature is surprisingly high. A single, separate 2-mL vial displayed a warming rate of +1.2 °C/sec, and singular 1-mL vials warmed at a rate of +2 °C/sec.5
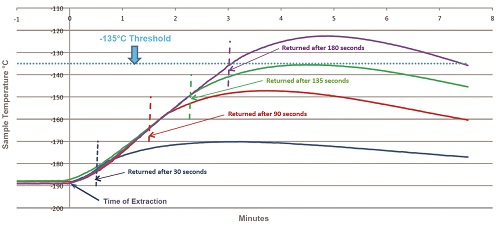
Figure 1 – Warming effect of short-term exposure of biosamples to ambient temperatures. In each scenario, temperatures were measured for three FluidX (Brooks Life Sciences, Indianapolis, IN) 2-mL vials filled with 1 mL water, placed in the corners of a 10 × 10 cryobox located on the top shelf of a cryogenic freezer rack. The freezer rack was removed from the –190 °C vapor-phased LN2 freezer for the specified durations. The threshold indicates the glass transition phase of water (Tg-H2O = –135 °C), below which all biological activity is thought to cease.
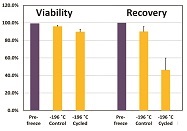
These warming effects are not without consequences. Recent experiments show that functionality of cryopreserved human mesenchymal stem cells (MSCs) is impacted dramatically by such TWEs, even when the sample never warmed to temperatures higher than –110 °C.3 An MSC control group (CG) remained for 67 days inside an LN2 freezer, whereas the MSC variable group (VG) was exposed to ambient temperatures 20 times, each –110 °C. In these tests, the time at ambient temperatures until the samples reached Tg-H2O was usually under 2 minutes. Downstream tests revealed a viable recovery rate of 87% for the CG MSCs, compared to prefreeze cell populations, whereas the VG MSCs had a viable recovery rate of only 41% (Figure 2). These rates remained similar after 13 months of MSC storage in LN2, and 70 TWEs.5
 Figure 2 – Viability and recovery of human MSCs after 67 days’ storage at –190 °C, with (cycled) or without (control) 20 TWEs to –110 °C.
Figure 2 – Viability and recovery of human MSCs after 67 days’ storage at –190 °C, with (cycled) or without (control) 20 TWEs to –110 °C.Cold-chain preservation during transport of low or medium numbers of biosamples
While avoidance of TWEs in everyday laboratory practice is not trivial, cold chain-of-custody management during the relocation of biomaterials can be even more challenging. Breaks in proper cold-chain maintenance of biomaterials happen most often during shorter transport processes, where researchers tend to resort to simple sample carriers filled with dry ice (–50 °C to –75 °C). However, this approach introduces temperature cycling that crosses Tg-H2O (LN2 to dry ice), can be unsafe, and is unstandardized. Alternative transport vessels have therefore been developed and should be employed for valuable cell-based biomaterials, even for the shortest transport routes. Such carriers (one example is depicted in Figure 3) are usually LN2-based and include a temperature-monitoring device to maintain an audit trail and alarm features if temperatures reach critical levels.5
 Figure 3 – Example of a safe, secure, LN2-based cryogenic sample carrier for short in-house transport of valuable biomaterials.5 The pictured unit, a CryoPod carrier (Brooks Life Sciences), maintains temperatures below –150 °C for over 4 hours.
Figure 3 – Example of a safe, secure, LN2-based cryogenic sample carrier for short in-house transport of valuable biomaterials.5 The pictured unit, a CryoPod carrier (Brooks Life Sciences), maintains temperatures below –150 °C for over 4 hours.For shipment of samples between facilities located in different cities, various sturdy metallic shipping containers filled with liquid nitrogen are available and go some way to ensure maintenance of the proper cryogenic temperatures. Care should be taken to provide enough liquid nitrogen in case of unforeseen delays. Dry vapor shippers, where the liquid nitrogen is compartmentalized and adsorbed into a specific material, are an even better option, and should include sensors that can continuously monitor and record the inside and outside temperatures during shipment, and thereby indicate whether the biomaterial had undergone undesired TWEs.
Relocation of entire sample collections or laboratories
What if the entire laboratory or biobank is moving? Failure to provide the correct temperature environment for the entirety of the samples of a laboratory or facility during a move would be catastrophic. This scenario requires specialized trucks or mobile biorepositories, vehicles that are outfitted with 110-V and 220-V electrical systems, alarmed temperature control and temperature monitoring, power generators, and the various electric plug conformations that are common in laboratory environ- ments. Importantly, the monitoring systems in these vehicles should conform to the regulations outlined in Title 21 of the Code of Federal Regulations, Part 11, to be able to verify storage temperature(s), an audit trail of the sample, and a full chain-of-custody of actions. Redundancy of power generation is key in these vehicles to ensure maintenance of the proper storage conditions for the biosamples. Self-leveling lift gates (Figure 4) are a desired feature on these trucks to minimize the risk of equipment damage.5
 Figure 4 – Self-leveling lift gate of a specialized truck for relocation of
Figure 4 – Self-leveling lift gate of a specialized truck for relocation of
laboratory freezers.
Several companies specialize in relocating temperature-sensitive materials, and considerations of the applicable certifications for safe transport of the specific materials (according to U.S. DOT regulations), and compliance with current Good Manufacturing Practices, should direct to the right service provider for each laboratory. Employment of an experienced lab relocation service provider guarantees a time-efficient and cost-effective move while maintaining the integrity of biosample collections.
Emergency transport of bulk quantities of biosamples
Disaster preparedness is essential for biobanks, biotech companies, and laboratories, and a business continuity plan should exist in case of natural disasters or other unforeseen events, including loss of power or equipment failures. Whereas in the latter two cases redundant generators may be able to ensure cold-chain maintenance of biosamples, emergency transport of bulk quantities of these biomaterials might be necessary after events that destroy the facility’s physical storage space, such as hurricanes, earthquakes, or floods.
Past experiences, such as those from a freezer outage at the Harvard-affiliated MacLean Hospital that damaged one-third of the largest collection of autism brain samples,6 showed that equipment failures can have a dramatic effect on sample viability, and ultimately affect the success of expensive and important research projects. In contrast, rescue operations in three medical centers in Manhattan, New York, after Hurricane Sandy in 2012 saved tens of thousands of biosamples from deterioration.7 During those emergency operations, samples were catalogued and transported in specifically equipped Relofleet mobile biorepositories (Brooks Life Sciences, formerly BioStorage Technologies) to backup storage facilities.
These events illustrate that contingency plans should include sample transport and relocation procedures, and that cold chain-of-custody requirements for stored biological samples should be addressed in those plans to ensure continued asset protection for the biobank, company, or research facility.
References
- Warhurst, J.; Fink, J. et al. Protection of Innocents: Continued Sample Warm up After Return to a Cryogenic Environment (Below–150 °C) Following a Transient Ambient Picking Operation (oral presentation). Annual International Society of Cellular Therapy Conference 2015, Las Vegas, NV.
- Hawkins, B.; Abazari, A. et al. Biopreservation best practices for regenerative medicine GMP manufacturing and focus on optimized biopreservation media. Cell & Gene Therapy Insights 2017, 3(5), 345–58.
- Fink, J. and Karnieli, O. The effect of common transient warming events on post thaw recovery and functionality of human mesenchymal stem cells stored in LN2 vapour environment. Cytotherapy 2017, 19(5), S112.
- Fink, J.; Meiring, A. et al. The effects of storage temperature and repetitive temperature cycling on the post thaw functionality of human mesenchymal stem cells. Cytotherapy 2016, 18(6), S45.
- Kunkel, E.J.; Comiso, S. et al. BioT™ cryopod carrier: standardized cryogenic temperature handling of cellular therapies. Cytotherapy 2015, 17(6), S31.
- Weintraub, K. Freezer failure at brain bank hampers autism research. The Boston Globe, June 11, 2012.
- Hager, R. Biobanking operations: contingency planning and disaster recovery of research samples. BioProcessing J. 2014, 13, 56–8.
Michael Lebbin is vice president of Transport Services, and Tina Middleton is marketing manager, Brooks Life Sciences, 22841 Torrance, CA 90501, U.S.A.; tel.: 866-977-2664; e-mail: [email protected] and [email protected]; www.brookslifesciences.com and www.brooks.com