Featured Article

Geothermal energy is a clean and renewable energy source with considerable potential for development. In China, low-temperature geothermal resources (≤90 °C) are relatively abundant compared with medium- (90–150 °C) and high-temperature resources (≥150 °C).1 While geothermal energy can be used for heating and other applications, a serious problem exists with pipeline scaling in the process of developing and transporting low-temperature geothermal fluid. A simple, economical, and effective solution to this problem is to add scale inhibitor.
Two traditional scale inhibitors—polyacrylic acid (PAA) and 2-phosphonobutane-1,2,4-tricarboxylic acid (PBTCA)—and two green biodegradable inhibitors—polyaspartic acid (PASP) and polyepoxysuccinic acid (PESA)—were selected for the scaling of CaCO3. As reported in the literature, one of the scale inhibitors has been studied over 80 °C.2,3 However, investigations have been lacking on the scale inhibition performance of the above inhibitors at 60–90 °C, the common temperature range of the low-temperature geothermal well. The current authors studied the scale inhibition performance of four scale inhibitors at different temperatures and dosages.
Experimental
The scale inhibition performance was analyzed with Fourier transform infrared (FTIR) and a scanning electron microscope (SEM). Testing was done using the static scale inhibition method according to the GB/T 16632-2008 standard.4 The four scale inhibitors used in this experiment are commercially available PBTCA (50%), PAA (≥30%), PESA (40%), and PASP (40%). These were diluted to 0.5 mg/mL. In the GB/T 16632-2008 standard, the heating temperature is 80 °C. To investigate the scale inhibition efficiency for geothermal fluids at different temperatures, the researchers changed the heating temperature from 60 °C to 90 °C; temperature gradient was 10 °C.
A solution containing 120 mg Ca2+ and 366 mg HCO3– was prepared. Four scale inhibitors were added in 500-mL conical flasks. A solution without inhibitors was also prepared. The conical flasks were heated at 60/70/80/90 °C for 10 hr (the level of solutions should not be higher than the water bath level). After heating, the solution was cooled to room temperature and filtered. Finally, the concentration of Ca2+ in the solutions was measured using EDTA standard solution. Performance of scale inhibitors was evaluated by the scale inhibition rate as calculated using Eq. (1):

(1)
Where ρ2 (mg/mL) is the concentration of Ca2+ in the solution with added scale inhibitors, ρ1 is the concentration of Ca2+ in the solution without scale inhibitors before heating in the water bath, and ρ0 is the concentration of Ca2+ in the solution without scale inhibitors after heating.
Results and discussion
Scale inhibition rate
Figure 1a–d shows the scale inhibition rates of PBTCA, PASP, PAA, and PESA with different additions at 60/70/80/90 °C, respectively. The scale inhibition rate of the four scale inhibitors increased with increase in dosage. Scale inhibitors at low dosage have poor scale inhibition performance, which is most pronounced in PESA. At 80 °C, compared with the blank group, there was no change in Ca2+ content of PESA with 2-, 4-, and 6-mg/L dosages after heating for 10 hr, and the scale inhibition rate was 0. At 90 °C, the scale inhibition rate was also close to 0 when the addition of PESA was 2 mg/L and 4 mg/L. At 60–90 °C, when the dosage of inhibitors was ≥6 mg/L, the scale inhibitor maintained a high and stable inhibition rate.
 Figure 1 – Scale inhibition rate of PAA, PBTCA, PASP, and PESA at different dosages at 60 ° (a), 70 ° (b), 80 ° (c), and 90 ° (d).
Figure 1 – Scale inhibition rate of PAA, PBTCA, PASP, and PESA at different dosages at 60 ° (a), 70 ° (b), 80 ° (c), and 90 ° (d).When the heating temperature was 60 °C, 70 °C, and 90 °C, the scale inhibition effects of the four scale inhibitors were PBTCA>PASP>PAA>PESA. However, the scale inhibition performance of PASP was greatly reduced at 80 °C. The scale inhibition performance of the four scale inhibitors was PBTCA>PAA>PASP>PESA, and the inhibition rate of PASP was only 1.71–15.38%. At 60 °C, when PBTCA dosages were 6 mg/L, 8 mg/L, and 10 mg/L, the scale inhibition rates were 64.44%, 67.13%, and 66.23%, respectively. PASP inhibition rate increased to 61.31% when the dosage was 8 mg/L. The scale inhibition rate of PBTCA and PASP at 70 °C increased to 81.82% and 73.74% when the dosage was 10 mg/L. PBTCA showed good scale inhibition performance at 80 °C. When the dosage was 10 mg/L, the scale inhibition rate was 64.1%. The highest scale inhibition rates of PAA, PASP, and PESA were only 48.72%, 15.38%, and 21.37%, respectively. At 90 °C, the scale inhibition rates of PBTCA and PASP were 65.73% and 61.54% when the dosage was 10 mg/L.
The above analysis demonstrates that the scale inhibition performance of PBTCA and PASP is better than PAA and PESA at 60 °C, 70 °C, and 90 °C. It is worth mentioning that PASP is a nontoxic, environmentally friendly scale inhibitor that was developed based on studying the metabolic processes of marine animals. It can be completely degraded into an end product that is harmless to the environment. PBTCA, on the other hand, is an organic phosphate, which easily forms into organic phosphoric acid scale. Considering the environmental impact, PASP is a better choice.
As can be seen in the above analysis, when the dosage of scale inhibitor is above 6 mg/L, the inhibition effect tends to be stable. The effect of temperature on the scale inhibition rate of the four inhibitors when the addition was 10 mg/L is shown in Figure 2. With the increase in temperature, the scale inhibition rate of PESA and PAA gradually decreased, and the scale inhibition rate at 90 °C decreased to 12.59% and 43.36%, respectively. The temperature resistance of PESA and PAA was poor, while PBTCA had good temperature resistance. Scale inhibition rates at 60/70/80/90 °C were 66.23%, 81.82%, 64.1%, and 65.73%, respectively. PASP also showed good temperature resistance at 60 °C, 70 °C, and 90 °C; scale inhibition rates were 56.38%, 73.74%, and 61.54%, respectively. However, the scale inhibition rate was only 15.38% at 80 °C. The reason for this will be the subject of future research.
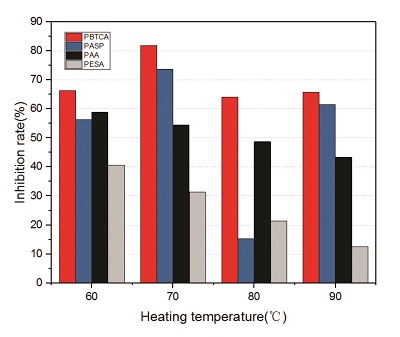 Figure 2 – Scale inhibition rate of 10-mg/L inhibitors at different temperatures.
Figure 2 – Scale inhibition rate of 10-mg/L inhibitors at different temperatures.Scaling inhibition mechanism of inhibitors FTIR
There are three common crystalline structures of CaCO3: calcite, aragonite, and is the most stable crystalline structure of CaCO3. Vaterite is the most unstable structure, and the stability of aragonite is somewhere in between.5
Figure 3 shows the FTIR spectra of precipitated scale products in the absence and presence of PAA, PESA, PBTCA, and PASP. The absorption peak of CaCO3 without scale inhibitor is 712 cm-1 and 876 cm-1, revealing that the main crystal structure of calcium carbonate is calcite.6,7 Except for the absorption peaks at 712 cm-1 and 876 cm-1, the characteristic band at ~1085 cm-1 was also found in the samples in the presence of PAA, PBTCA, and PESA; ~1085 cm-1 is a characteristic band of vaterite.6,7 This confirmed that calcite has the tendency to transform into vaterite in the presence of PAA, PBTCA, or PESA. However, the current authors did not find the characteristic band of vaterite in the FTIR analysis of the CaCO3 sample with the addition of PASP, so they used SEM for further analysis.
 Figure 3 – FTIR analysis of CaCO3 samples in the absence and presence of PAA, PESA, PBTCA, and PASP.
Figure 3 – FTIR analysis of CaCO3 samples in the absence and presence of PAA, PESA, PBTCA, and PASP.Figure 4a and b is an SEM image of CaCO3 without inhibitor, and 4c–f shows CaCO3 with PAA, PBTCA, PESA, or PASP. The three crystal structures of CaCO3 have different microstructures—the morphology of calcite is cubic, aragonite is acicular, and vaterite is spherical.8,9
 Figure 4 – SEM graphs of CaCO3 samples with different scale inhibitors: CaCO3 without inhibitor (a, b), PAA-CaCO3 (c), PBTCA-CaCO3 (d), PESA-CaCO3 (e), and PASP-CaCO3 (f).
Figure 4 – SEM graphs of CaCO3 samples with different scale inhibitors: CaCO3 without inhibitor (a, b), PAA-CaCO3 (c), PBTCA-CaCO3 (d), PESA-CaCO3 (e), and PASP-CaCO3 (f).
When the scale inhibitor was not added to the system (Figure 4a and b), the crystal structure of CaCO3 was mainly regular hexahedron, and crystal size was about 5–7 μm. The structure cell stacks closely and the shape is regular. This is a typical calcite structure. In the SEM analysis of the CaCO3 sample with PAA (Figure 4c), the morphology of the crystals changed—the original hexagonal structure gradually lost its edges and corners. The CaCO3 sample with PBTCA (Figure 4d) shows that the crystal surface gradually became smooth and the corners almost disappeared. Crystal size is about 3 μm. The CaCO3 scale sample with PESA (Figure 4e) has no hexagonal structure. Its scale structure is loose with no obvious angularity. Compared with the blank group, the above results confirm that the addition of PAA, PESA, or PBTCA causes CaCO3 to transform from calcite to vaterite, which is consistent with the FTIR results.
Unlike the other three scale inhibitors, a large number of acicular structures with a length of about 20 μm was observed in the CaCO3 scale sample in the presence of PASP (Figure 4f). This is a crystal structure of CaCO3 between calcite and vaterite called aragonite. Compared to the stable cubic structures of calcite, aragonite is also an unstable crystal structure of CaCO3. At the same time, this also explains the phenomenon that the FTIR image of CaCO3 adding PASP does not show the characteristic peak of vaterite.
Conclusion
Increasing the dosage of the scale inhibitor and heating temperature causes the scale inhibition rate to increase, and, when the dosage is ≥6 mg/L, the four scale inhibitors achieve stable inhibition performance. At 60 °C, 70 °C, and 90 °C, the scale inhibition efficiency is PBTCA>PASP>PAA>PESA; at 80 °C, PBTCA>PAA>PASP>PESA. PBTCA is an efficient scale inhibitor with good temperature resistance. Taking into account environmental factors, PASP is a better choice at 60 °C, 70 °C, and 90 °C.
Based on the results of FTIR and SEM analyses, the scale inhibition mechanisms of the four scale inhibitors can be summarized as follows: the addition of scale inhibitors changes the CaCO3 structure from dense and stable calcite to loose, irregular change in crystal morphology makes CaCO3 less likely to adhere to the surface of the device and is more likely to be present in water in a suspended state, making it easier for circulat- ing water to wash away the calcium scale.
References
- Lin, W.J.; Liu, Z.M. et al. The assessment of geothermal resources potential of China. Geology in China 2013, 40, 312–21.
- Zheng, Y.F.; Yang, L.J. et al. Research progress on environment friendly scale inhibition. J. Environ. Man., College of China 2013, 23, 50–2.
- Wang, W.J.; Liu, X.H. et al. On synthesis of green polyethylene succinic acid derivatives and their scale inhibition. J. Tangshan College 2015, 28, 68–72.
- Chinese National Standard GB/T 16632- 2008. Determination of the scale inhibition performance of water treatment agent-calcium carbonate deposition method, 2008.
- Lei, Y. Research progress of vaterite type calcium carbonate. J. Yangtze University 2014, 11, 35–9.
- Menzri, R.; Ghizellaoui, S. et al. Calcium carbonate inhibition by green inhibitors: thiamine and pyridoxine. Desalination 2017, 404, 147–54.
- Xyla, A.G.; Mikroyannidis, J. et al. The inhibition of calcium carbonate precipitation in aqueous media by organophosphorus compounds. J. Colloid Interface Sci. 1992, 153, 537–51.
- Rautaray, D.; Sainkar, S.R. et al. Thermally evaporated aerosol of thin films as templates for the room temperature synthesis of aragonite crystals. Chem. of Mater. 2003, 15, 2809–14.
- Chen, J.C.; Li, H. et al. Research on the performance of different scale inhibitors and their effect on CaCO3 crystal form. Technol. of Water Treatment 2017, 43, 76–9.
Wenxi Zhu, Xiuhua Zheng, and corresponding author Guomin Li are with the School of Engineering and Technology, China University of Geosciences (Beijing), Beijing 100083, P.R. China; e-mail: [email protected]; http://en.cug.edu.cn. Dehua Hu is with the School of Analysis and Test Center, Beijing Normal University, Beijing 100875, P.R. China. The authors would like to thank the National Natural Science Foundation of China projects, “Design and Preparation of Green Environmental Friendly of Self-Degradable Foamed Temporary Cementitious Sealing Materials for Geothermal Reservoir” (grant no. 41572361) and Fundamental Research Funds for the Central Universities (grant no. 2652017068) for supporting this research and allowing the authors to publish this document. They would also like to thank the Key Laboratory of Deep Geodrilling Technology of Ministry of Natural Resources and the Demonstration Center for Experimental Geological Resources Exploration Education.