The novel SARS-CoV-2 virus has presented researchers around the world with a huge challenge- to characterize the virus in record speed. Since the outbreak, the scientific community has come together in both academia and industry to accelerate the development of efficient diagnostics and treatments for COVID-19. However, virus research is incredibly challenging due to the difficulty of characterizing particles with a diameter of less than 250 nm. Fortunately, single-molecule and super-resolution imaging can provide valuable insights into, for instance, viral structure, infection mechanism, and receptor binding and thus facilitate the identification of potential targets for antivirals.
Virus sizes range between 20-250 nm, which appear as blurry spots when imaged on conventional light microscopes that are diffraction-limited. Until recently, electron microscopy was the technique of choice for virus research, but the high cost, complex sample preparation, and the difficulty in identifying the location of a specific biomolecule greatly limits its usefulness for virus characterization. While standard fluorescent microscopes allow the direct identification and visualization of specific proteins and biomolecules by using a combination of antibody and fluorophores, its low resolution prevents a detailed understanding of the virus itself and virus-cell interactions.
Single-Molecule Localization Microscopy (SMLM) is a super-resolution imaging technique that overcomes the diffraction limit of light, enabling imaging down to 20 nm resolution. This level of detail allows researchers to conduct in-depth investigations into how viruses are assembled, how different components are arranged, and how viruses interact with cells. For example, we can gain insights into receptor-virus binding kinetics and how viruses are assembled.
Achieving 20 nm Resolution Using SMLM
In fluorescence microscopy, the emitted light from a fluorophore appears as a blurry spot (~200 nm in diameter) due to the diffraction of light through the microscope, termed the diffraction barrier. When multiple fluorophores are imaged at once, as in a typical wide-field microscope, these blurry spots overlap with one another making it impossible to distinguish one fluorophore from another.
The idea in SMLM is to excite a small portion of the fluorophores in the sample at a given time to remove the overlapping signals which caused the image to appear blurry.This is achieved by making fluorophores blink so that the emission from each fluorophore can be spatially and temporally separated. The process is sequentially repeated many times until most fluorophores in the sample have been imaged. At the end of the acquisition, a reconstructed image is formed.
One of the most commonly used SMLM techniques is direct Stochastic Optical Reconstruction Microscopy (dSTORM). dSTORM takes advantage of the photoswitchable properties of organic dyes to achieve the stochastic activation of fluorophores, Figure 1a. Fluorophores are made to blink by manipulating their electron state, turning them either ON (fluorescent) or OFF (dark). Fluorophores can be turned OFF by using high laser power and an oxygen-scavenging and reducing buffer. The fluorophores can then be turned back ON by irradiation with an activation UV laser or by reaction with oxygen.

Figure 1. Super-resolution imaging of viruses. a. Principles of super-resolution. b. Comparison of TEM, standard microscopy, and dSTORM. dSTORM image of viral surface glycoprotein (magenta) and cellular host factors (cyan) in an HIV-reconstructed virus. (Melikian Lab, Emory University, Atlanta, USA)
Single-Molecule Techniques for Virus Research
dSTORM is an ideal technique for studying viruses because it combines high-resolution with the ability to visualize key biomolecules. The HIV particle shown in Figure 1b, has been labeled with antibodies to visualize surface glycoprotein (magenta, AF647) and cellular host factors incorporated into the particle (cyan, AF488). The dSTORM image appears just like any other fluorescence image except now with the nanostructure of the sample revealed.
Besides imaging the structure of viruses, dSTORM can be applied to gain mechanistic insights into how viruses infect cells and into which cellular machinery it hijacks for replication, Figure 2. For example, when the Ebola virus infects a cell, it completely reorganizes the actin cytoskeleton network. The virus then reshapes and uses actin to nucleate inclusion bodies which allows the virus to replicate efficiently. Furthermore, the transport of the assembled virus is mediated by actin. Within a single diffraction-limited spot, dSTORM can resolve and distinguish between the assembly and transport of the Ebola virus.
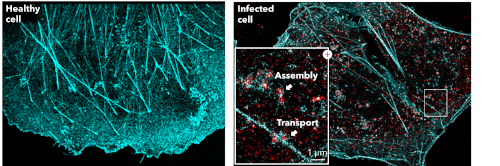
Figure 2. Super-resolution imaging of viruses and viral infection. dSTORM image of actin stained with phalloidin-AF647 (cyan) and NP-AF55 (red) in Ebola virus VLP transfected cells. (Becker lab, Marburg University, Germany)
dSTORM is a powerful method to access structural and interaction information at a biological relevant magnification. However, the resulting image is a snap-shot in time and does not provide us insights into the dynamic nature of the viral cycle. To address this, another technique, single-particle tracking (SPT) can be used to visualize and quantify the movement of the virus protein of interest, Figure 3. The mobility of a molecule, expressed here by its diffusion coefficient, gives us insight into the molecule’s immediate environment and can thus indicate whether the molecule is experiencing random, confined/restricted, or a directed transport. A decrease in the diffusion rate can imply binding or interaction of the labeled molecule with another molecule or subcellular structure. Furthermore, SPT can be extended to 2-color imaging to simultaneously track two molecular species and determine their dynamic interaction or preferential spatial localization.
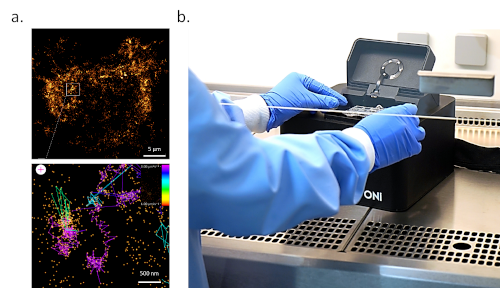
Figure 3. Real-time imaging of virus assembly sites. a. Single-particle tracking of influenza HA (HA-dendra) in HEK293T cells. The tracks are colored by their diffusion coefficient. (Chiantia Lab, Potsdam University, Germany) b. ONI’s Nanoimager is a desktop microscope capable of dSTORM and SPT imaging. It can easily be used in space-limited laboratories, including BSL3 and BSL4 facilities.
SPT enables spatiotemporal dynamic localization of viral particles, e.g. during assembly and maturation, envelopment, and release. Figure 3 presents an example of the influenza HA protein labeled with Dendra in HEK293T cells. The color of the track indicates the diffusion coefficient of that protein. The virus assembly sites can be distinguished due to their low diffusion coefficient and confined motion.
Virus Imaging Within Your Biosafety Cabinet
A major concern with virus research is the safety of the researcher. Live virus research can only be performed under strict safety conditions, forcing scientists to resort to studying attenuated viruses or virus-like particles (VLPs) instead of intact viruses. Often it is unclear whether the conclusions drawn from VLP experiments can also be applied to real viruses and, therefore, further validation steps are required. Luckily, ONI’s Nanoimager, a small benchtop super-resolution microscope, offers a unique, compact design that makes it an ideal tool to support research performed in space-limited laboratories, including BSL3 safety cabinets and BSL4 facilities, without the need for additional infrastructure such as optical tables or dark rooms, Figure 3b. This would allow researchers to study unperturbed viruses, test antiviral efficiency directly, and, thus, accelerate the research and therapeutic development process.
In conclusion, the study of viruses requires a detailed understanding of their structural diversity and the mechanisms by which they are able to infect cells and spread. Single-molecule techniques are therefore essential for the development of diagnostic tools, vaccines, and antiviral treatments.
Rebecca C. Gilson, Ph.D., is a Field Application Scientist at ONI ([email protected]) and Linnea Olofsson, Ph.D., is a Application Scientist at ONI ([email protected])