Cryopreservation is the preservation of living and structurally intact cells and tissues by cooling to very low temperatures, in some cases as low as -196°C. It has widespread medical applications, such as in fertility treatment, bone marrow transplantation and the storage of red blood cells (RBCs) for transfusions, as well as for many biological research purposes. Living cells cannot be stored using simple cooling or freezing methods for prolonged periods of time due to processes such as ice crystal formation, osmotic shock, and membrane damage during the freeze-thaw cycle, which ultimately results in cell death1. Successful cryopreservation depends on an understanding of the physical and chemical properties occurring during freezing and thawing, as well as the appropriate use of cryoprotective agents (CPAs) and temperature control tools.
A key challenge in cryopreserving materials is keeping the cells alive after cooling and rewarming for use. Intracellular ice formation (IIF) is the primary cause of death of cells cryopreserved in solution. IIF is characterised as the formation of ice crystals within cells during rapid freezing, and is dependent on factors such as cooling rate, which must be better understood and carefully controlled to maintain cell integrity during cryopreservation.
Considerable research efforts have been made to characterise IIF mechanisms. Ice is known to form in extracellular water first when cells are frozen in solution, which leads to an increase in extracellular solution concentration, disrupting the chemical potential between water inside and outside the cells2. Consequently, water either passes out of the cell via the plasma membrane through osmosis and the cell dehydrates, or it forms ice within the cell (IIF), both of which cause cell damage. The two stages of IIF – ice nucleation and ice crystal growth – are the subject of ongoing intensive study, made possible by continuous developments in cryomicroscopy technology.
The first commercially available cryomicroscope was launched in 1985, but researchers had been developing their own systems ten years prior, which could control freezing and thawing in biological cell suspensions to observe them at sub-zero temperatures. The development of temperature-controllable cryostages has since enabled the control over temperature accuracy and cooling/warming rates, and they are often compatible with a range of optical microscope models – facilitating the knowledge exchange critical for cryopreservation research.
Investigating IIF Mechanisms
Researchers at the University of Alberta are investigating how IIF during cryopreservation is affected by the degree of supercooling and cell volume, in the absence of CPAs. By using cryomicroscopy, the group could record the formation of intracellular ice in individual human umbilical vein endothelial cells (HUVECs), and link this to cell volume and the amount of solution supercooling3. Intracellular supercooling, cell volume, and extracellular nucleation temperature have shown to be important parameters affecting the nucleation of intracellular ice.
Using cryomicroscopy (Nikon Eclipse 80i microscope, Linkam FDCS196 stage), HUVECs were cooled to temperatures that gave specific degrees of intracellular cooling, then extracellular ice was nucleated and the incidence of IIF was evaluated. Here, the cryomicroscope offers a beneficial experimental system for IIF studies because it enables visualisation of the cells as they are subjected to sub-zero temperatures and extracellular ice nucleation. IIF can be detected by the darkening of cells, and cell survival after thawing was determined using a membrane integrity assay (Figure 1).
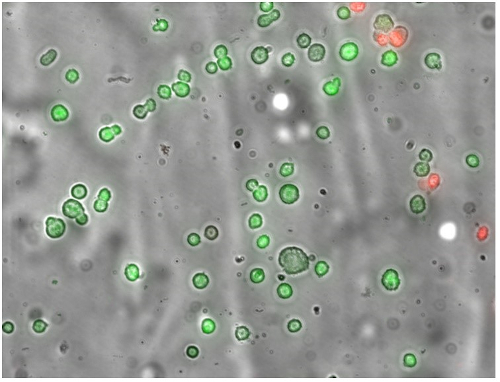
Figure 1: Representative image of SYTO®13 (green)/EB (red) fluorescence used for membrane integrity assay. Green cells have intact membranes and red cells have damaged membranes. Reproduced from reference 3 in accordance with the Create Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/.
This technique facilitates direct cell-by-cell comparison of various parameters, such as IIF, cell volume, and post-thaw membrane integrity. The cryostage uses a small sample volume, which enables near-instantaneous dissipation of the latent heat of fusion. This keeps cells at the desired sub-zero temperatures and prevents rebound to the freezing point, which is a known issue with larger sample volumes.
These experiments reveal a correlation between increased intracellular supercooling and more intracellular ice formation, which happens as the ice nucleation temperature decreases (in solutions with the same osmolality). It was also shown that cells with a smaller diameter before extracellular ice nucleation have a decreased chance of IIF, and the study demonstrated that these smaller cells can withstand more supercooling before experiencing IIF. Such findings could be used to inform cryopreservation protocols.
Optimising RBC Cryopreservation
A group at the University of Ottawa used cryomicroscopy to assess the ability of ice recrystallisation inhibitors to improve cryopreservation and post-thaw recovery for human RBCs4. Many CPAs, such as glycerol, are useful cryoprotectants but are toxic above certain concentrations, so current cryopreservation protocols do not allow the direct transfusion of RBCs immediately after thawing. In Canada and the United States, clinical RBC cryopreservation protocols use high concentrations of glycerol (>40% wt./vol.) with slow cooling rates (1°C/min.). To prevent post-transfusion intravascular haemolysis, complex and time-consuming post-thaw deglycerolisation procedures are needed to ensure glycerol concentrations are reduced to less than 1% prior to transfusion, which poses a key challenge that limits the use of cryopreserved RBCs in transfusion medicine.
The issues posed by glycerol have led to the assessment of alternative CPAs for RBC cryopreservation, including non-penetrating additives and simple carbohydrates and oligosaccharides. Researchers at the University of Ottowa investigated the ability of novel small molecule phenolic-glycosides to inhibit ice recrystallisation and freeze RBCs with reduced glycerol concentrations with slow freezing rates. It was shown that a phenolic-glycoside ᴅ-glucose bearing a β-linked para-methoxyphenyl (PMP) moiety (β-PMP-Glc) could be used to freeze RBCs using slow cooling rates and 15% glycerol, through significantly decreasing post-thaw RBC haemolysis, conferring protection during both the slow cooling and rapid cooling/storage at -80°C stages of the freezing protocol.
Data also suggested that β-PMP-Glc might provide protection against cryo-injury by interacting with the RBC membrane and/or penetrating the cell. Cryomicroscopy experiments confirmed that ice structure during the RBC freezing process is significantly different when β-PMP-Glc is present (Figure 2). This is the first example of a novel small molecule ice recrystallisation inhibitor influencing ice structure in the presence of RBCs in vitro.

Figure 2: Ice crystal and cryomicroscopy images using an Eclipse 80i microscope (Nikon) and a FDCS196 cryostage (Linkam). Photographs illustrating the ice recrystallisation inhibitor activity of β-PMP-Glc (6) and the advancing ice-front during RBC freezing. Advancing ice fronts during RBC freezing in the presence of (A) 110 mM β-PMP-Glc + 15% glycerol solution, (B) 110 mM β-PMP-Glc in dextrose/saline solution, or (C) 15% glycerol solution. Reproduced from reference 4 in accordance with the Create Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/.
Improving Cryopreservation in the Future
The results from these two studies provide a promising backdrop to advancing understanding of IIF, and to improve cryopreservation protocols for a range of clinical research and biomedical uses. The ability to control the temperature of samples under the microscope using cryostages, such as the Linkam FDCS196 stage, has significantly developed the potential of cryomicroscopy, and through this technology parameters such as cell volume have been confirmed to affect the probability of IIF. This, alongside the discovery of new ice recrystallisation inhibitors, sets the scene for continued innovation in the field of cryopreservation.
Duncan Stacey is the Sales and Marketing Director of Linkam Scientific Instruments.
References
- Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, Choi CW, Lee SR, and Han J (2017) Cryopreservation and its clinical applications, Integr Med Res, 6(1):12–18.
- Yi J, Liang XM, Zhao G, and He X (2014) An Improved Model for Nucleation-Limited Ice Formation in Living Cells during Freezing, PLoS ONE, 9(5): e98132.
- Prickett RC, Marquez-Curtis LA, Elliott JAW, and McGann LE (2015) Effect of supercooling and cell volume on intracellular ice formation, Cryobiology, 70: 156-163.
- Capicciotti C, Kurach JDR, Turner TR, Mancini RS, Acker JP, and Ben RN (2015) Small Molecule Ice Recrystallization Inhibitors Enable Freezing of Human Red Blood Cells with Reduced Glycerol Concentrations, Scientific Reports, 5: 9692.