by Jackie Trudell, Portfolio Marketing Manager Chromatography and Mass Spectrometry
PerkinElmer
High performance liquid chromatography (HPLC) has been utilized in a wide range of applications in the food, industrial and pharmaceutical industries for decades, offering robust and straightforward testing and analysis capabilities for expert and novice users alike.
However, as the demand for faster separations, better peak resolution and lower detection limits has increased in a number of industries, many labs have begun to transition their HPLC methods to ultra-high-performance liquid chromatography (UHPLC) instruments. As this evolution continues from HPLC to UHPLC, led initially by pharmaceutical laboratories with others in various industries starting to follow suit, it is helpful to understand the basic differences between the two techniques, the key benefits UHPLC can bring, and best practices and factors to be considered when transferring methods to ensure consistency and data quality.
A Quick Primer HPLC vs. UHPLC
The configuration of the pump and column are primarily what differentiates an HPLC system from a UHPLC system. HPLC pumps typically deliver solvent at consistent pressures between 6,000 and 10,000 psi (413 – 689 bar). It is imperative that the pump pressure be maintained at a constant rate, even with the backpressure created as the mobile phase interacts with the stationary phase. A constant flow rate, along with built-in solvent degassers to remove dissolved gasses in the mobile phase, ensure reproducible and accurate results.
HPLC columns typically contain particles that are 3-5 µm in size, and vary in length and diameter, depending upon the intended application. Although not always the case, longer column formats typically yield better separations, as non-target compounds are exposed to the column for longer periods of time. Longer columns, however, slow down the analytical process, and it is thus important to balance separation efficiency with the need for fast separations.
UHPLC columns, on the other hand, utilize smaller particles, typically 2 µm or less. As the particle size is reduced, so is the size of the column itself. UHPLC columns are often half the size of HPLC columns in terms of both diameter and length, increasing the efficiency of the separation, and thus the speed of the analysis. Studies have shown that it is possible to achieve an equivalent level of chromatographic efficiency utilizing a 150 mm HPLC column packed with 5 µm particles, and a 50 mm UHPLC column packed with sub 2 µm particles1. The sample analysis time, however, is improved 9-fold using UHPLC1. The smaller particles used in UHPLC columns also yield narrower chromatographic peaks, improving resolution and sensitivity.
However, it’s important to note that to accommodate UHPLC columns with smaller particle sizes, UHPLC pumps must be able to operate at a higher pressure to propel the mobile phase through the more tightly packed stationary phase. UHPLC pumps commonly offer solvent delivery at pressures in the 12,000 – 18,000 psi (827 – 1241 bar) range. The higher pressures associated with UHPLC pumps can cause column degradation owing to the pressure shock on the column experienced during sample injection as the valve switches. Recent technology, such as PerkinElmer’s Intermediate Loop Decompression (ILD™) injection valve, reduces these pressure fluctuations, thus increasing column lifetimes, even at the higher pressures generated by UHPLC columns.
Why Transfer a Method from HPLC to UHPLC?
Cost is often a major concern for commercial laboratories, and the initial cost of a UHPLC system is often higher than an HPLC system. This cost, however, can often be quickly balanced by the increased throughput labs can achieve by using UHPLC. That being said, labs running a smaller number of samples may not be able to appreciate this cost savings – although, if the analytical results are time-sensitive, UHPLC can provide a much faster time to decision turnaround, so a cost benefit can be produced in other ways.
The cost savings and benefits associated with UHPLC don’t end there. Owing to the increased efficiency of smaller particle size columns and the higher back pressure, solvent flow rate is reduced, resulting in less solvent consumption during analysis. Reducing solvent consumption not only lowers the cost of each sample analyzed, but also minimizes the waste generated during analysis, which can help companies work towards their corporate social responsibility goals.
Best Practices for Method Transferability from HPLC to UHPLC
Once the decision to transfer methods from HPLC to UHPLC has been made, a number of factors should be considered to ensure method transfer is efficiently and effectively completed.
Choosing the Right Analysis
Not all analytical methods are inherently good candidates for method transfer to UHPLC and may not yield sufficient financial or efficiency gains to support the inherent costs associated with implementing new methods. Good candidates for migration to UHPLC are methods with long run times (≥20 minutes), those that use reverse phase columns, as well as methods or labs with heavy sample loads.
Choosing the Right Column
When transferring a method to UHPLC, ideally, the same type of column should be utilized to maintain the analyte retention order and ensure consistency over time. The optimal inner diameter of a UHPLC column is around 2.1 mm, as this column size reduces mobile phase consumption and frictional heating, which can lead to column degradation. Taking time to choose the right UHPLC column will simplify the migration from the HPLC method. As a general “rule of thumb,” the following conversions can be used when transferring from a 4.6 mm internal diameter, 5 µm particle HPLC column:

Figure 1: Column Dimension Transfer Guidelines
- Mobile Phase Flow Rate: The flow rate (F) for the UHPLC method must be adjusted to maintain a mobile phase linear velocity (u) similar to what was used in the HPLC method. The linear velocity within a column is directly proportional to the column diameter (dc), but also depends on the particle size (dp) of the stationary phase. Therefore u*dp must be maintained at a constant value to account for changes in column diameter and particle size.

- Isocratic Step Time: The ratio between the isocratic step time (tiso) and the column dead time (which depends on the mobile phase flow rate (F), column diameter (dc) and length (L)) must be maintained between the HPLC and UHPLC conditions.

- Gradient Slope and Step: As a best practice, the initial and final mobile phase compositions in any HPLC gradient step should be maintained in the UHPLC method. The slope and time of the gradient step in the UHPLC method must be adjusted such that the product of the gradient slope and the dead time remain constant between the HPLC method and the UHPLC method. The UHPLC gradient slope (slope2) can be calculated using the following formula:

To illustrate the transfer of a method from HPLC to UHPLC, consider the analysis of ginsenosides of ginseng2. The HPLC and UHPLC column and instrument details are displayed in Table 1.

The injection volume for the UHPLC method (was decreased 14-fold:
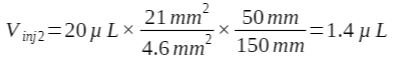
Next, the UHPLC mobile phase flow rate is calculated at 0.82 mL/min, decreasing by a factor of 1.8.

The isocratic step time was reduced from 7 minutes to 0.89 minutes utilizing the UHPLC method.

Calculating the gradient slope and slope time of the UHPLC method yields:

In the example above, the gradient slope should be increased 9-fold.
Chromatograms from the HPLC and UHPLC analysis of ginsenosides are presented in Figure 2.

Taking the above calculations into account, the transfer of the method for ginsenosides of ginseng from HPLC to UHPLC resulted in a 91% decrease in mobile phase consumption. Further, the runtime for the analysis was shortened by a factor of 5.
Conclusion
The transfer of a method from HPLC to UHPLC can yield significant productivity improvements and cost savings, owing to a reduction in run time and solvent consumption. These cost savings and throughput improvements can quickly balance the increased cost of capital associated with UHPLC instrumentation.
References
- Guillarme, D., Veuthey, P. (2008). Requirements for UHPLC instruments, method Development in UHPLC and Method Transfer from Regular HPLC to UHPLC (pp. 1-9, Tech.). Waltham, MA: PerkinElmer
- Pedjie, N. (2011). Analysis of Ginsenosides in Ginseng Root with the PerkinElmer Flexar FX-15 System Equipped with a PDA Detector (pp. 1-3, Tech.). Shelton, CT: PerkinElmer