
Titration is used in the laboratory to determine the concentration of an analyte in a solution. This process involves the addition of precise incremental volumes of a liquid titrant solution from a burette (in volumetric titration) or application of an electrical current of precise magnitude (in coulometric titration) to induce a chemical reaction; when the reaction is complete, the total volume of the titrant added, or time that the current was applied, can be used to calculate the concentration of the analyte.
Titration can be performed manually or using automated titration instruments, and there are various types of titration based on the analyte, titrant and chemical reaction used, as well as the method used to indicate the equivalence point and endpoint of the reaction. This article will focus on the chemical reactions used to reach an equivalence point between the analyte and titrant.
The chemical properties of the sample determine the best titrant to use for concentration analysis, as the titrant will need to react specifically with the analyte to reach a detectable equivalence point (the point at which all of the analyte has reacted with the titrant). The four main types of titration based on the chemical reaction used are acid-base, oxidation-reduction (redox), precipitation and complexometric titration.
Acid-Base Titration
In acid-base titration, the analyte is an acid and the titrant is a base, or vice versa. This results in a neutralization reaction, in which the solution approaches a neutral pH (7) as the titrant is added. Typically, a strong acid or base is used as the titrant, such as hydrochloric acid (HCl) or sodium hydroxide (NaOH), respectively. While these reagents are more hazardous, they allow for a fast reaction and ensure the analyte can be fully neutralized.1
The pH of the solution at the equivalence point will differ depending on the strengths of the acids and bases that are reacting. When a strong acid reacts with a strong base, the solution will be neutral (pH 7) at the equivalence point, while the solution will be acidic when a strong acid reacts with a weak base, and basic when a strong base reacts with a weak acid. The equivalence point pH for a reaction between a weak acid and weak base depends on which is stronger, and will be neutral if they are of equal strength. This is most important to consider when using a color-changing indicator for the titration.
Acid-base titration is versatile because it can be used to titrate virtually any acidic or basic analyte. In addition to being able to determine the concentration of an analyte with a known acid or base dissociation constant (pKa or pKb), it can also be used to determine an unknown pKa or pKb for an analyte of known concentration in a solution. However, acid-base titration is less suitable for very weak acids and bases, and may not be applicable to sample mixtures containing other acids or bases that can interfere with the reaction between the titrant and target analyte.
Redox Titration
In oxidation-reduction (redox) titration, the analyte is an oxidizing agent and the titrant is a reducing agent, or vice versa. Redox titrations can be direct or indirect, with indirect titrations involving an additional step in which the product of a redox reaction with the analyte is titrated, rather than the analyte itself. For example, iodine can be used to directly titrate some reducing agents, becoming iodide when reduced, in a titration process known as iodimetry, while in the indirect process known as iodometry, an oxidizing analyte first reacts with iodide to produce iodine, which is then titrated back into iodide using a reducing agent such as sodium thiosulphate.
Various titrants and procedures can be used for redox titrations, with potassium permanganate being a popular oxidizing titrant for analytes such as hydrogen peroxide, oxalates and ferrous compounds, and iodometry being commonly used to measuring oxidizing agents, such as dissolved oxygen or chlorine, in a solution. Karl Fischer (KF) titration, one of the most widely used methods to determine water content, involves a redox reaction in which H2O is consumed when iodine oxidizes sulfur dioxide, although the H2O does not directly oxidize or reduce the other chemicals. KF titration can either be volumetric, in which the volume of iodine added is used to calculate water content, or coulometric, in which amount of charge from a current that converts potassium iodide to iodine is used to calculate the H2O consumed.
In some cases, redox titration can be much more specific than acid-base titration, due to the specific oxidation/reduction of certain analytes by certain titrants. However, there is a tradeoff to this specificity, as there are more limitations in selecting titrants that will react effectively with the analyte. Additionally, the pH of the sample solution may need to be adjusted to ensure a successful reaction, and evaporation of volatile reaction components (such as iodine) can be a source of error. For manual titrations, some redox titrations do not require a separate indicator due to the color changes that naturally occur as a result of the reaction.
Precipitation Titration
In precipitation titration, the titrant and analyte react to form an insoluble precipitate. This method can be direct or indirect, and is most commonly used to measure salt content (sodium chloride) and other halide ions such as bromide and other chlorides. Silver nitrate is typically used as the titrant and forms silver halide precipitates in the reaction. While highly specific, precipitation titration is limited to analytes that can form an insoluble precipitate with the titrant. Silver nitrate is also relatively expensive compared with other common titrants.
Complexometric Titration
In complexometric titration, the titrant forms a complex with the analyte; this type of titration is used to measure the concentration of metal ions in a sample solution. Ethylenediaminetetraacetic acid (EDTA) is the most common titrant used in complexometric titrations. While complexometric titration is valuable for measuring metal ion concentrations, especially for mixtures of different metal ions, it can be more difficult to automate, as doing so typically may require optical equipment such as a colorimeter or spectrophotometer to read the color change of a complexometric indicator. However, complexometric titrations can be performed with some automatic titrators using potentiometric or thermometric methods.
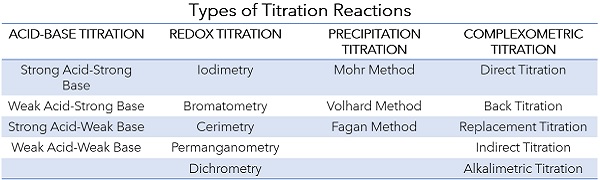
Figure 1: The Four Main Type of Titration Reactions
References
- Lower, S. 13.5: Acid/Base Titration. In Chem1 Virtual Textbook.; LibreTexts Chemistry; Open Education Resource LibreTexts Project, 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Chem1_(Lower)/13%3A_Acid-Base_Equilibria/13.05%3A_Acid_Base_Titration