Water is a critical reagent in the laboratory and contains several types of impurities and contaminants—such as particulates, organics, inorganics, microorganisms, and pyrogens—that can adversely affect testing results. These contaminants and impurities must be removed by running the feed water through a water purification system.
Purchasing the right water purification system is a major step. Below are the factors that must be considered in the selection process.
Feed water
The source of the feed water affects the type and level of impurities in the water. If the feed water is from ground water, impurities may include dissolved organic and inorganic compounds derived from pesticides, fertilizers, and detergents. Feed water from underground feed water may have a high level of hardness, but low levels of organics.
An understanding of the feed water source and the level of impurities in that source will help determine how much purification is needed to reach the level of purity required for a specific laboratory activity.
Water purity
Because impurities can be a critical factor in many research applications, the level of water purity ranks high in importance. Therefore, several organizations have established a grading system for purified water.
The American Society for Testing and Materials (ASTM) has established uniform standards of water purity. These can be seen in Table 1 in this article. The most common breakdown of the types of water can be seen in Table 2—Types I* through III—with Type I* being the purest. Applications for purified water can be seen in Tables 2 and 3, respectively, in the same article.
Figure 1 shows a water infographic to help match the research laboratory application with the correct water type, and thus aid in the selection of a water purification system.
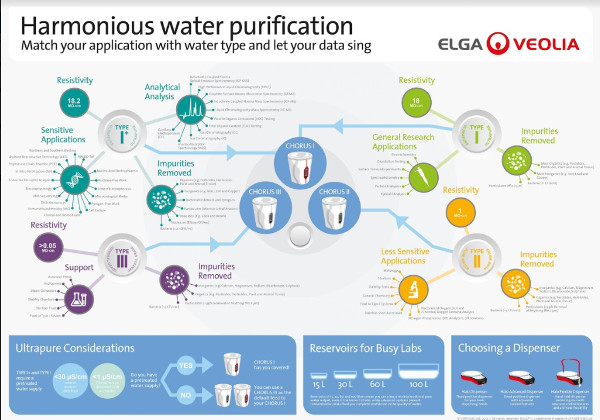 Figure 1 – Image courtesy of Elga LabWater.
Figure 1 – Image courtesy of Elga LabWater.Purification techniques
Several water purification techniques are available. For example, ion exchange is needed for removing inorganics, and highly organic compounds as well as dissolved gasses such as CO2. Suspended particles and colloids require ultra- or microporous filtration, and reverse osmosis is essential for the removal of dissolved organic and inorganic compounds. Electrodeionization removes ionic charged articles, including inorganic and organic compounds.
Table 1 lists the types of purification techniques to select from to achieve the desired application sensitivity, the levels of resistivity, total organic carbons, filtrates in the water, bacteria, endotoxins, nucleases, and the water grade and water type.
 Table 1 – Purification techniques
Table 1 – Purification techniques*Flame/AAS: flame/atomic absorption spectrometry; GC/MS: gas chromatography/mass spectrometry; GF/AAS: graphite furnace/atomic absorption spectrometry; HPLC: high-performance liquid chromatography; ICP/AES: inductively coupled plasma/atomic emission spectrometry; ICP/MS: inductively coupled/plasma/mass spectrometry; TOC: total organic carbon
Consistency
While all water purification systems may produce the highest purity of water, it is important to select those with the features that ensure high-quality purified water is produced consistently.
Water usage
Instantaneous as well as daily water volume requirements should be thought through ahead of time; for variable water demand, flexibility and modularity are also important considerations. Therefore, it is important to know in advance how much water will be used per hour, per day, or per week, and the locations in the laboratory in which the purified water will be used. As shown in Figure 1, the type of research application will determine the location and extent of water usage.
Long-term needs
The laboratory must consider whether its needs will change in the future, a determination that may impact the choice of water purification system. Future needs may be related to the potential for variability in application sensitivity, and the level of resistivity, total organic carbon, the amount of filtrates, bacteria, endotoxins, and nucleases, and finally a changing requirement for water grade and water type.
Budgeting
Budgeting should be generally considered at the point of purchase to ensure not only the ability to purchase the water purification system, but also the ability to buy supplies and accessories involved in operation of the system, and to maintain a preventive maintenance schedule to ensure that the system remains operational.
System features
Important features to look for in a water purification system are:
- Footprint and weight
- Wall-mount, bench, or floor installation
- Facility for hands-free operation or by foot pedal
- Availability of built-in life-cycle alarms and calibrators
- System monitoring and compatibility with Windows or Mac operating systems
- Availability of a USB connection, PIN-coded access to software, or data capture
- Real-time total organic carbon monitoring
- Auto-volume dispense, variable flow rate dispense, and locked dispense
- Purity monitoring to point of use.
System benefits
In addition to having certain features that make the water purification system functional for its intended purpose, other benefits, such as the psychological and aesthetic appeal of the system, may be important. These include accessibility of the water purification system to its users without being obtrusive, and the option and ability to place system components under a bench, on the wall, or in a different laboratory, thus leaving room for the other parts of the research laboratory to operate.
Conclusion
There are many factors to consider when selecting a water purification system. Having an appreciation for the level of water purity required for a specific research application will help determine how much purification is needed, as well as avoid the use of too much pure water, which could lead to waste and increased purification costs.
More information on water purification systems can be found at http://www.elgalabwater.com.
Lina Genovesi, Ph.D., JD, is a technical, regulatory, and business writer based in Princeton, NJ, U.S.A.; e-mail: [email protected]; www.linagenovesi.com