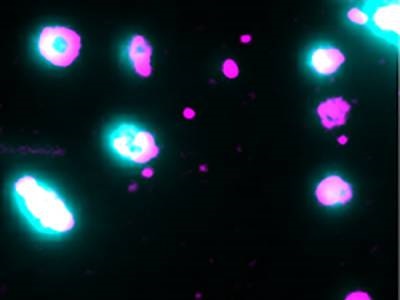
Liposomes are loaded with dye (cyan) to pin-point opening of the pores by membrane-bound pore-forming proteins (magenta). Single-molecule microscopy captures the moment the pores open and the dye is released. Credit: James Walsh, UNSW Syndney
Pore-forming proteins are used by various organisms to attack and damage the membranes of other cells, whether it be immune cells destroying diseased cells or bacteria gaining entry to a host. Several types of pore-forming proteins have been identified, but exactly how individual protein molecules assemble to form a pore has remained largely unknown due to the difficulty of tracking such tiny structures. Now, researchers from the European Molecular Biology Laboratory (EMBL) Australia Node in Single Molecular Science at the University of New South Wales have developed a single-molecule microscopy technique to monitor these specific proteins, revealing new details about the pore formation pathway of perfringolysin O.
The researchers’ method involved the use of purified proteins and liposomes as a simple model system representing the target membrane. The liposomes were loaded with fluorescent dye that allowed the researchers to pinpoint the exact moment a pore opened and released the dye. This approach, combined with super-resolution total internal reflection fluorescence (TIRF) microscopy, provided sufficient resolution and sensitivity to track the entire pore formation process in real-time, allowing the team to visualize each step in the process as individual protein molecules assembled together, said corresponding author Till Böcking.
The experiments revealed that once two perfringolysin O molecules binded together on the liposome membrane, this stable interaction triggered the process of adding more protein subunits to form a ring structure. They additionally found that a pore opening could be created even before the full ring formed – they observed some pore openings occurring after just four protein subunits were assembled. Insertion rate was found to increase linearly depending on both the number of subunits in the oligomer and the concentration of perfringolysin O in the solution, the authors wrote. Furthermore, pore formation activity of perfringolysin O was impaired at pH levels above 7. These revelations about the assembly pathway of perfringolysin O, which is secreted by Clostridium perfringens as a bacterial virulence factor, provides scientists with valuable new information for designing strategies to target specific pore-forming stages and potentially modulate their activity. This research was published in eLife.
“The take home message is that these are very efficient hole-punching machines that have evolved to form pores in all sorts of circumstances,” said Böcking. “We hope that understanding that the conditions actually dictate the assembly pathway gets people to rethink the way they do their experiments.”
The researchers believe that the mechanisms they observed in perfringolysin O may be similar for other cholesterol-dependent cytolysins, which could be the subject of further research. Additionally, the study showed the value of the single-molecule TIRF microscopy method for observing the activity of pore-forming proteins.