Modern pharmaceuticals frequently rely on cell cultures grown in bioreactors or fermenters as part of large-scale manufacturing lines. This is made possible by the development of prototype processes using benchtop equipment to create controllable environments for research and regulatory approval. At the heart of this equipment is control over the gases that influence fermentation and optimize performance.
Pharmaceutical production is a highly regulated business that relies on precision manufacturing techniques using state-of-the-art equipment developed over many years. Data recording and repeatability are essential for gaining and maintaining strict regulatory compliance, and the development work completed in the lab heavily influences these outcomes.
To understand how this can be optimised, it is important to look at the process as a whole and the effects of various influences. The practice of fermentation is thousands of years old, used to produce bread, cheese, and yoghurt as well as alcoholic drinks. Today, biotechnology based on the same principles is used to manufacture everything from fuels to chemical solvents and cosmetics as well as biopharmaceutical medicines.
Modern Biotechnology
Biotechnology is key in developing active pharmaceutical ingredients (API) for modern cancer drugs, vaccines, antibiotics, insulins, or hormones such as erythropoietin (EPO). Bacteria or cell cultures are used as a biocatalyst. The ideal growth of such microorganisms is achieved by bioreactors or fermenters, which carefully control the process (Figure 1).

Figure 1: Bioreactor with modular gas control station. (Source: Bürkert Fluid Control Systems)
Compared to chemical production processes, biotechnology is less efficient, simply because bioprocesses usually take a much longer time and the product yield is comparatively low. The great advantage is that only biotechnology enables the production of many products with complex molecular structures, a key requirement for the production of important pharmaceuticals.
These processes are based on biological cell growth using microorganisms as biocatalysts and these have a specific time within which the population will increase twofold, known as the doubling time. The fermentation processes for biopharmaceuticals use bacteria, yeast or mammalian cell cultures, each of which has its own special characteristics.
Organism Choices
The foundation of a good fermentation process is the use of a suitable growth media, which must provide the perfect nutrients for the specific microorganism. Typically, this contains water, sugars such as glucose, salts, and micronutrients.
The growth of bacteria, such as Escherichia coli (E. coli) is generally fast with a doubling time of around 30 - 60 minutes and requires simple growth media that is cost-effective, enabling a simple and rapid fermentation process. These features make bacteria ideal for high volume production. Bacteria fermentation is limited to simple organic compounds, like hormones or amino acids. The products are generated inside the cell, which requires elaborate downstream processing to release the desired product from the cells. A famous final product of bacteria fermentation is insulin.
Yeasts have many characteristics in common with bacteria in that they are not overly sensitive to environmental conditions and they are used to produce hormones, amino acids or simple proteins. Attachment of carbohydrate chains to the proteins is possible but not as good as if mammalian cells are used, causing risk of immune reactions.
Mammalian cells, such as Chinese hamster ovary (CHO) cell cultures, are widely used for producing complex proteins such as antibodies. The biotechnological production of antibodies revolutionized medicine by offering the possibility to produce passive vaccines, cancer drugs, and drugs for autoimmune disorders. However, cell growth is slow, around one to two days doubling time, and the cultures are very sensitive to environmental conditions, resulting in a slow and complicated fermentation process. In addition, the growth media for cell culture fermentation is usually complex and very expensive, making each batch a valuable one.
Fermentation
The fermentation process for pharmaceuticals always takes place inside a bioreactor, which is the general name for any equipment containing a biocatalyst and is usually used for small scale fermentation processes. A fermenter is a specific type of bioreactor enabling a controlled growth atmosphere. Commonly, the term fermenter is used for large scale fermentation processes.
The bioreactor enables ideal cell cultivation, since the environmental conditions can be controlled precisely, enabling an optimum growth rate of the biomass. This stage is known as upstream processing. The fermentation begins by inoculating the growth media with the microorganism of interest. In the lag phase or incubation phase, the microorganisms adapt to the new environment and the cell growth is slow.
Following this, the exponential phase starts with a steady increase of growth rate. Eventually, the growth rate slows down during the deceleration phase, due to the falling concentration of nutrients. This is followed by the stationary phase, where the biomass stays constant before it enters the death phase, where the microorganisms start to die (Figure 2). For optimum efficiency, the bioprocess should be stopped before entering the stationary phase.
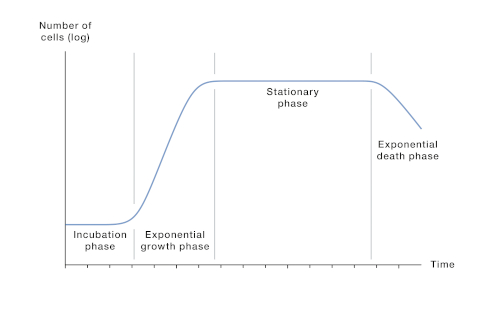
Figure 2: Phases of a fermentation process. (Source: Bürkert Fluid Control Systems)
Bioreactors and fermenters are available as batch, fed-batch and continuous processing versions. Fed-batch and continuous fermentation enable longer exponential growth phases and thus a higher yield, but require extreme caution in avoiding contamination. The laboratory fermentation is typically a pure batch process. The most common designs are stirred tank reactors (STR) where the tank is made from glass or single-use plastic bags. The single-use equipment has the advantage that it comes completely sterile and does not need sterilization in an autoclave after each batch.
Gas Control
Within a bioreactor or fermenter, the environmental conditions must be optimized. The temperature in the fermenter is controlled precisely using electric heaters or heating jackets, while the pH value and the dissolved oxygen concentration are controlled by gassing systems.
In all, there are four different gases used for environmental control. Air, oxygen (O2), nitrogen (N2) and carbon dioxide (CO2). Oxygen increases the dissolved oxygen concentration, which represents the “food” for aerobic microorganisms. N2 is used for the so called “stripping”, which means the removal of O2 and CO2 from the liquid in order to decrease the acidity and thus increase the pH value. For anaerobic microorganisms, the nitrogen is required for the cells themselves. The CO2 is used to lower the pH value and provides carbon as an energy source for the cells.
However, pure CO2, N2 or O2 can often negatively impact the bioprocess. Therefore, for bacteria or yeast fermentation, which is very robust, usually a standard air supply is sufficient for the fermentation, since it contains enough N2 and O2 for good process control. For cell culture growths, the additional (unwanted) constituents of air are not acceptable. In this case, the pure gases are mixed to create the necessary gas composition.
The transfer of the gases into the fermentation broth can be achieved by sparging, where the gas supply is located at the bottom of the tank, and overlay, where the gas is introduced from the headspace. Sparging is used for maximum oxygen transfer and it has an additional stirring effect. Overlay is designed for a smoother gas transfer into the liquid (Figure 3).

Figure 3: Setup of a bioreactor with gas supply for overlay and sparging. (Source: Bürkert Fluid Control Systems)
Precision Management
The requirements of the gas control system are particularly extensive for pharmaceutical cell culture fermentation. The cell cultures are extremely sensitive and the growths can be negatively impacted if the environmental conditions inside the bioreactor are not perfect. Each fermentation process takes a long time, typically a few weeks, and the growth media as well as the microorganisms are extremely expensive. If the dissolved oxygen levels, for example, are incorrect, the microorganisms can die easily and the entire batch is ruined.
This means that the gas control equipment needs to have high accuracy and repeatability to optimize the cell culture growth. It also requires high turndown ratios (control range) of the gases to keep up with the changing flow requirements during the exponential growth stage and/or the use of the same equipment for different reactor sizes. In addition, rapid flow control is required to ensure any disturbances are managed promptly.
Bürkert’s mass flow controllers (MFC) fulfil these requirements by using the proven thermal measurement principle, with direct sensors that take measurements in the gas stream for the fastest control times, accuracy and repeatability as well as very high turndown ratios. The inbuilt, direct-acting control valves have a tight shutoff, which makes addition shutoff valves redundant. MFC types 874x cover a wide flow rate range, starting at a few milliliters up to 2500 l/min. Another major advantage of direct sensors is the long-term stability of the flow calibration. Frequent recalibrations are usually not necessary, which significantly reduces the effort in service and maintenance, and therefore the overall runtime costs.
Of course, for pharmaceutical applications, there is a requirement for all wetted parts to comply with USP Class VI (Chapter 87 & 88) and often high-quality stainless-steel bodies are required. As an experienced supplier to the biopharmaceutical sector, all of Bürkert’s products, including MFCs and (control or shutoff) valves can be specified in a range of materials to suit each application (Figure 4).
Bespoke Design
As with any pharmaceutical application, there is a requirement to record critical process parameters (CPP) and prove the consistency and reliability of the critical quality attributes (CQA). Bürkert MFCs provide not only the precise mass flow control data, but on top of that also valuable diagnostics, like the device status, a flow totalizer and alarm messages. All the process and device data can be logged and transferred to the superior control systems using a variety of communication protocols, making integration much simpler.
By working closely with OEMs, Bürkert offers a comprehensive design and build solution that tailors each component to the specific needs of the bioreactor or fermentor. Bürkert MFCs cover a wide flow range, which supports the easy scaling up from benchtop laboratory systems to pilot reactors and finally production scale fermenters (Figure 5). Each system can be built, tested and certified prior to delivery to ensure optimized performance and dependability.

Figure 4: Bürket system solution, including mass flow controllers and solenoid valve bank for gas mixing. (Source: Bürkert Fluid Control Systems)

Figure 5: Producing a large enough cell quantity to inoculate a production fermenter by means of seed train scaling up. (Source: Bürkert Fluid Control Systems)
About the Author: Lukas Hammer has a Master of Science in Physics from the University of Munich. Since 2013 Product Manager for MFCs at Bürkert Fluid Control Systems. Special focus on bioreactors, since one of the biggest growing MFC markets.