Ion Mobility Spectrometry (IMS) burst forth like a supernova in the Waters hospitality suite of the ASMS 2019 meeting. One can think of IMS as gas-phase electrophoresis where the ion passes through the gas impeded by collisional resistance which is dependent on the shape of the analyte ion. Thus, it is particularly useful in discriminating between isomers including anomers. IMS has been around for decades, but Waters added an orthogonal cyclic ion mobility device with a one-meter long pathlength coupled to a newly designed quadrupole time of flight mass spectrometer (QToF MS) that will certainly expand research interest in IMS of biologics.
Plus, out on the poster floor, I also found a novel ion mobility spectrometer from Ion Dx Inc. (Monterey, CA) designed for measuring properties of biopolymers including antibodies and potentially protein isomers responsible for BSE and Alzheimer’s disease. For additional information regarding ion mobility spectrometry review the article: Ion Mobility Spectrometry for Determination of Native Structure of Antibody-Based Drug Candidates.
Waters Introduces the SELECT SERIES Cyclic IMS Instrument
Waters has employed ion mobility spectrometry platforms ahead of their mass spectrometers for a decade or so. The length of the IMS stage was 25.4 cm which gave some separation based upon differential mobility of ions, but for some analytes the differences were small. Increased ion mobility resolution was required. One way to address this would be to build a longer ion mobility separator; but how long was long enough 2 m? 5 m? 20 m? As a novel alternative, Team Waters designed a racetrack that would provide an indefinite pathlength in a compact design (Figure 1). This novel platform has been incorporated into a research-grade time-of-flight mass spectrometer and is called the SELECT SERIES Cyclic IMS instrument. It is a floor standing instrument about the size of a refrigerator. Large, yes, but a refrigerator-size is much smaller and more practical than an instrument with a linear ion mobility platform of multi-meter length.

Figure 1: Schematic of the path of ions in Waters Select Series of Cyclic IMS Spectrometer. Ions from a capillary enter the left-hand T wave trap which collimates the ion cloud and passes it on to the Stacked Ring Ion Guide (SRIG). The ions quickly travel through the high energy collision cell and into the switching region, where ions are stored, diverted to the cyclic IMS stage, or passed directly to the ToF mass spectrometer.
The ion path in figure 1 proceeds from left to right. Sample ions are formed in an electrospray ion source (ESI) (a) left and enter the StepWave which transmits charged analyte ions and strips out uncharged molecules. The ion guide (IG yellow) shapes the cloud and delivers the ions to the quadrupole mass filter which can be used to select ions for a targeted m/z. The ions are subsequently collected and accumulated in a trap cell. These ions are again efficiently focused by the stacked ring ion guide (IG gray). Ions are released from the trap and passthrough the helium gate to ease the ions into the high-pressure region of the ion mobility separation stage, continuing through the pre-Store region of the cyclic IMS (figure 1 B & C) and into the multi-function array. Typically, this region is about 2 millibar of nitrogen gas. The cyclic IMS assembly is perpendicular to the main axis of the ion optics.
Traveling waves in the cyclic IMS stage propels the ions around the circular array. The first pass has a 98 cm drift length with a resolution (Ω/ΔΩ ) of 65. Each successive pass adds ~1 meter to the path and improves the resolution Ω/ΔΩ= A(nz)^0.5. where n=number of cycles and A is the single-pass resolution (Ω/ΔΩ).
The versatile cIM stage can be programmed to capture, store and separate ions using customized instrument control software. Ions separated by the cyclic ion mobility device are mass measured using the reflectron TOF mass spectrometer, with an offset V or W Geometry. This TOF mass analyzer has a resolution of higher than 60,0000 (FWHM). Instrument control and data analysis is provided by Waters’ MassLynx and the new Mobility Miner Software.
Applications of cIM
Cyclic IM is bound to create curiosity. Many, including stock market analysts, will try to forecast the impact in business and science. Waters enjoys the resources and experience to quickly establish demand. Examples include the introduction of Q-Tof and ACQUITY UPLC, and other innovative products in mass spectrometry, thermal analysis, and especially liquid chromatography. With something as new and innovative as cyclic IMS, Waters needs to answer the question: “What are the limits?” The second question is “Can I… Should I use it in my research?”
Responding to this challenge, team Waters presented more than ten posters reporting results of cIM. A few are summarized below.
Higher-order Structure of Native Proteins by IMS
One of many posters from Waters Corporation authored by Dale A. Cooper-Shepard and colleagues showed how the collision-induced unfolding of proteins can reveal details of protein structure.
The authors selected to study the unfolding of hTTR (human Transthyretin protein) which is a tetrameric 56 kDa protein complex often used as an example for native MS studies. The sample was electro sprayed from a 2 m i.d. glass tip (New Objective). Native ion mobility spectrometry was performed on the cyclic IM instrument. The details of protein folding and unfolding are relevant to the higher-order structure of proteins and the progression of protein diseases. With the cIM instrument, it is possible to follow the unfolding of native proteins. The key enabling technology is the functionality of the Cyclic IMS, with the key technology and modes of operation highlighted in the center of Figure 2.
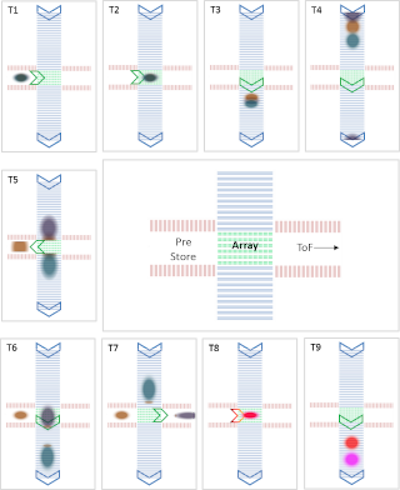
Figure 2: Schematic diagram showing the use of the CIM interface for real-time control of the ions. Ions can be sorted, stored, fragmented and analyzed in one run. Copyright © 2019 Waters Corporation.
The geometry of the cyclic IM enabled multi-stage gas-phase separations to be performed allowing the stepwise unfolding of the TTR complex to be studied. Through precise timing of the traveling waves on the multifunctional array, unfolded protein ions can be selectively ejected into the pre-array store where they were held briefly before reinjection of these ions into the cIM device for an additional pass.
This study reminds me of the numerous liquid phase electrophoretic separations. If cIM could replace them, the market for the instrument could be immense. One question is: are gas-phase data relevant to liquid phase conditions. In some (many?) cases, the answer is probably yes.
Details of MS Fragmentation of Benzocaine
A poster by Professor Jim Scrivens and colleagues of Teesside University, (Middlesbrough, UK) used the cyclic ion mobility enabled mass spectrometer for qualitative detection and quantitative analysis. The particular problem was the location of the proton on protonated benzocaine (BH+). The different protomers correlate with two different fragmentation pathways and give rise to different fragment ions. Such information may be useful in predicting the fragmentation of ions in multiple reaction monitoring for LC-MS/MS.
The authors point out that the cIM offers several benefits:
1. The circular path provides a selectable, extended flight path which improves resolution as a square root function, with each lap around the circle. A single pass of the device provides a baseline resolution of 65 (CCS/ delta CCS). Higher-resolution separations are achieved by selecting the target ions and then resolving them using multiple passes around the circular path.
2. Each pass around the circle increases the drift path by 98cm. Resolution of the ions increases with the n ^0.5, but the circular design minimizes the instrument size and consumption of drift gas which is usually purified nitrogen at about 2 millibar pressure.
3. Transit time around the IMS track is only a few milliseconds. Run time is about 2 seconds.
4. A segmented quadrupole transfer stage connects the cIM and TOF stages shapes the ion beam before entrance to the TOF mass analyzer. The TOF stage is capable of resolution of 100,000 @ FWHM. Dynamic range is 10^6.
The very interesting poster concludes that the rotational average collisional cross sections () are within about 4% of with prior reports using orthogonal techniques. After 12 cycles, two protomers of the [M+H]+ ion are observed with a resolution (/) of 225. Depending upon the location of the charge, the different protomers produce different fragmentation patterns.
cIM of Penta Saccharide Conformers
Carbohydrate microheterogeneity is heavily involved in protein higher-order structure (HOS). Important details include stereochemistry and anomeric configuration. These are important in biological mechanisms including signaling, infections and immune response. Polysaccharides are also the key structural elements of plants including development.
Traditionally, NMR has been used to characterize carbohydrates, but as the carbohydrates become more complex, spectral complexity and ambiguity increase even faster. LC-MS can help define the primary and secondary structure, however, determining higher-order structures can be difficult. Additionally, the highly charged ions produced by ESI can induce structure change through charge-charge repulsion.
A poster from Jakub Ujima and colleagues at Waters Corporation (Wilmslow, UK) described the use of cyclic IMS-MS for differentiating between the anomeric forms of Penta saccharides. The authors first demonstrated the increase in resolution obtained from multiple passes for three Penta saccharides. The first pass had a resolution of ~ 65, which gave two broad peaks. After 5 passes, the resolution had increased to about 145, with three main peaks clearly separated in approximately 47 milliseconds.
Next, they examined three Penta saccharides using IMS/IMS technology. Each standard had three peaks. The authors injected the precursor ion into the pre-store region. Subsequent reinjection of the stored ions with raised activation energy allowed fragments to be produced and then separated by IM (Figure 1). The fragmentation patterns were able to define the conformational details of the isomers. The authors conclude that IMS/IMS-MS technology is useful in the differentiation of reducing end conformers (anomers and open-ring) and also metal ion binding sites.
Lipids
Lipids are often complex, labile molecules. Understanding their structure and function is increasingly important in elucidating disease mechanisms. Lipid signaling compounds are involved in signaling, structure, and storage within living organisms. Lipids are the subject of intense investigation, increasingly via LC-MS/MS. However, the work is difficult due to the structural diversity of isobaric analytes. Adding cIM to HPLC-MS increases the peak capacity and information content of discovery protocols. This can potentially resolve cis/trans configurations and positional isomers of fatty acids.
In this preliminary study, three-component mixtures of signaling lipids including unsaturated fatty acids, steroids, and prostaglandins were infused into the cIM instrument. Initial results for unsaturated fatty acids showed that cis forms were more compact than the corresponding trans-isomers.
Steroids were more difficult. One sample of 11-deoxycortisol, 21-deoxycortisol, and corticosterone were only partially resolved after the first pass. However, after 12 cycles, 21-deoxycortisol was clearly separated from the two remaining standards. After twenty cycles the other two were partially resolved.
UPLC-cIM-MS
The high speed and orthogonality of cIM/MS naturally leads to the question of where does LC fit in? Team Waters provided an intriguing practical answer in a poster by Martin Palmer and colleagues.
The authors compared cIMS alone with UPLC of peptides combined with cIM detection. They found:
1. cIM was useful in detecting peptides from protein digests. The short (< 1 sec) run times for cIM improved peak capacity which can improve resolution or shorten the chromatographic run time to 3 minutes. Thus, multidimensional LC may find that LC-cIM-QToF is faster and more powerful.
2. Infusion-only cIM runs were comparable to UPLC runs in the number of observed peptides, with ~ 90% coverage of protein sequence.
3. Deuterium labeling as in deuterium exchange is compatible with cIM since slow-exchanging amino acid backbone deuteration is maintained.
Peak Tracking in Chromatography
Still another poster from Waters illustrates the utility of using the collisional cross-section (CCS) of ions for compound identification. Collisional cross sections (CCS) of ions are characteristic of the 3-dimensional shape of the ion in the gas phase. Waters has an application note showing that CCS is highly beneficial in matching peaks when comparing two complex chromatograms obtained under conditions where the elution order of the peaks is variable.
Summary
Waters has designed and introduced a novel next-generation ion mobility platform that is an exciting improvement in ion mobility technology. Team Waters has demonstrated utility in a variety of applications studies including proteins, peptides, lipids, polysaccharides, and small molecule drugs. It adds an orthogonal dimension to liquid chromatography and mass spectrometry, providing increased separation power. It will be interesting to watch the uptake by the research community.
References and Credits
1. Dale A. Cooper-Shepherd, et al. “Tandem Ion Mobility Coupled with Mass Spectrometry for Gas Phase Unfolding Studies. Waters Corporation, Wilmslow, Cheshire, UK. ASMS (2019) Poster Th 275.
2. James Scrivens, et Al. “Resolution and Characterization of Protomer and Radical Cation Species Utilizing a Cyclic Ion Mobility Enabled Quadrupole, Time-of-Flight Q-cIM-oaTof) Mass spectrometer. ASMS 2019 poster ThP302.
3. Jakub Ujma, et Al., “Cyclic Ion Mobility Mass Spectrometry Distinguishes Anomers and Open-Ring Forms of Penta Saccharides, J. Am. Soc. of Mass Spetrometr. (2019) 30:1028-1037. DOI: 10.1007/s13361-019-02168-9
4. Michael McCullagh, et Al., “Analysis of Lipid Signaling Class Analytes Using a Traveling Wave Cyclic Ion Separator”, ASMS 2019. Waters Poster Library PSTR135023275
5. Martin Palmer, et Al. “Application of cyclic ion mobility mass spectrometry for High Peak Capacity Separations of Native and Deuterated Peptide Mixtures.” Waters Corp. Wilmslow, UK. Poster, ASMS 2019 Waters Poster Library PSTR135024779 (2019)
6. Catherine Holdsworth, et Al. “Utilization of Ion Mobility Enabled Collision Cross Section Measurements for the Comparison of Metabolites Across Differing Chromatographic Methods” Waters Poster Library PSTR134917233 (2016)