Type 1 ultrapure water is by far the purest substance used in a laboratory. This article discusses the high level of purity of type I ultrapure water, the importance of such purity and how to maintain this standard during normal laboratory use.
Introduction
In today’s laboratories the availability of pure water for research and testing applications is essential. Elements and compounds present in the parts per billion (ppb) range or lower could negatively affect applications by interacting with samples, active media or system components.
One hundred per cent pure water consists solely of water molecules in equilibrium with hydroxyl and hydrogen ions (10-7M at 25ºC). This composition gives a characteristic electrical resistivity of 18.2 Mohm.cm. However, the unique ability of water to dissolve virtually every chemical compound to some extent and to support practically every form of life means that its purity is constantly under threat from five types of impurities. These are suspended particles; inorganic compounds; organic molecules; dissolved gases; and microorganisms including their associated biomolecules. To generate high purity water for laboratory use, mains drinking water is put through a series of purification steps to remove these different types of impurities.
The purity of type I ultrapure water
The recorded levels of impurities in type I ultrapure water are limited primarily by the sensitivity of the techniques available to measure these and the environment in which the testing takes place. On the basis of current ultra-trace techniques, the maximum levels of non-gaseous impurities present in type I ultrapure water are less than 1.5 μg/l (ppb) for organic compounds and less than 1.0 μg/l for other elements and ions. This means that type I ultrapure water is at least 99.99999975 % pure.
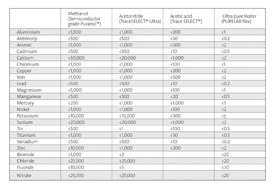
Table 1: Comparison of Elemental Impurity Specifications of Type I Ultrapure water and Top Grades of Common Solvents
Table 1 compares the elemental impurity specifications of type I ultrapure water with those of the purest grades of three commercially available solvents used widely in analytical research and testing applications: methanol, acetonitrile and acetic acid. Using the latest ICP-MS techniques, all non-gaseous elements were shown to be effectively absent from type I ultrapure water, most with detection limits of less than 1 ng/l (ppt). This level of contamination is orders of magnitude less than all the other solvents tested. Most other laboratory reagents have far higher levels of impurities than these solvents, frequently in the mg/l range.

Table 2: Volatile organic compounds by Purge & Trap GC-MS

Table 3: Semi-volatile compounds by thermal-desorption GC-MS
Tables 2 and 3 show the very low levels of volatile and semi-volatile organic impurities in type I ultrapure water, measured using purge and trap GC-MS and thermal-desorption GC-MS, respectively. Typically, levels of impurities are all below the limits of detection, <0.05 μg/l for volatile organic compounds and <0.025 μg/l for semi-volatile ones. These levels are consistent with a total organic carbon (TOC) value of less than 1μg/l (ppb), where TOC is an overall indicator of organic contamination often given for type I ultrapure water.
Type I ultrapure water does contain dissolved oxygen and nitrogen at around 9 ppm and 14 ppm, respectively, at 25ºC and 1 bar atmospheric pressure. These could be largely removed by vacuum degassing, however, this procedure is not necessary for most laboratory applications, as the moment that type I ultrapure water comes into contact with air, oxygen and nitrogen dissolve in the water at concentrations in equilibrium with the atmosphere.
Reverse osmosis and sub-micron and/or ultra-filtration remove all particles and bacteria from type I ultrapure water and endotoxins are removed by ion-exchange and charged media or ultra-filtration. Bacterial testing shows that type I ultrapure water contains <1 Colony Forming Units (CFU)/10ml, equivalent to <0.1 μg/l TOC.
Why use Type I Ultrapure water?
Although it may seem extreme to use high purity water, type I ultrapure water does need to be free from all these impurities if it is to be used across the whole range of analytical and experimental applications. Fortunately, this is an economical prospect, with type I ultrapure water only costing around ten pence per litre including all purchase and running costs.

Figure1: The effects of water impurities on the ion chromatography technique: (a) effects on the system and (b) the subsequent potential impact on experimental results. The area of the box indicates the significance of the impact (qualitative).
As water may be used in many aspects of an analysis, including preparation of samples, dilutions, standards, and blanks, as eluents and for rinsing instruments, the presence of any contaminants can compromise results. Figure 1 shows the numerous ways in which different impurities can affect the reliability and reproducibility of ion chromatography results both in the short and the long term.

Figure 2: Improvements in background for HPLC with UV detection at
210mm using type I ultrapure water with very low TOC

Figure 3: Ultra-trace cation analysis pre-concentrating 20ml samples
High sensitivity analyses are particularly dependent on the high purity of the water, especially when very low concentrations must be measured directly or when only very small samples are available and these need diluting before analysis. Using type I ultrapure water minimises background levels, enabling researchers to obtain highly sensitive results in trace analyses; for example using HPLC, see figure 2 (Reference A) or ion chromatography, see figure 3.
Maintaining purity
Laboratory workers requiring pure water must bear in mind that the purity of type I ultrapure water can easily become compromised before use if the system itself is inadequately managed or if the water is incorrectly handled during collection and usage.
Of primary importance is maintaining the water purity within the water purifier. The recommended way to achieve this is by protecting the water reservoir from external contamination using a composite vent filter, recirculating the water periodically through the final purification technologies, such as UV photo-oxidation, adsorption and ion-exchange, and regularly sanitising the system as required to minimise bacterial growth (Reference B).

Figure 4: Effect of exposure to air on resistivity of type I ultrapure water
In normal laboratory use, water is dispensed from a purifier, such as the PURELAB flex, into a vessel. Within seconds the water starts to absorb carbon dioxide from the air forming carbonic acid and reducing the resistivity of the water from 18.2 Mohm.cm to a minimum of about 1.3 Mohm.cm, see figure 4. The high conductance of the hydrogen ions from the acid enables this large change to be achieved at a CO2 concentration of only 0.5 mg/l. Though this carbon dioxide does not degrade the water for most applications, its effect on resistivity can mask the contamination of the water by other ions.

Figure 5: GC-MS of type I ultrapure water: effect of plasticizer in tubing
Much more significant for most applications is the risk of contamination as water is dispensed. For example, fixing a length of flexible plastic tubing to purifiers with fixed tap dispensers to make it more convenient to fill carboys or other large containers can cause the water to become contaminated. Figure 5 illustrates how organic release agents or plasticisers from the tubing can leach into the water: GC-MS scans show that type I ultrapure water passed through flexible PVC tubing can be contaminated with N-butyl sulphonamide plasticiser. In addition, a survey of users in a pharmaceutical company, showed that the average total viable bacterial count (TVC) in water from 22 water purifiers without tubing fitted was 0.7 CFU/ml but that this rose to 26 CFU/ml for seven units with additional tubing on the dispense.

Figure 6: Negative ion chromatography example of contamination from the atmosphere due to differences in water collection method: (a) water collected with splashing; (b) water collected by flowing it along the vessel i.e. no splashing
It is also important that air entrainment is minimised when type I ultrapure water is dispensed, as impurities in the air can also reduce the water’s purity. Figure 6 (Reference C) compares the contamination from the atmosphere between samples of water collected with splashing (lower trace a) and water collected so that it flowed along the wall of the vessel (higher trace b). Analysis using ion chromatography clearly showed that negative ions, particularly nitrite ions, were detected at higher concentrations when splashing had occurred.

Figure 7: Examples of phthalate ester contamination from wash-bottles (3).
To maximise its purity, type I ultrapure water should be used soon after it is dispensed. In a survey of wash-bottle use, Kuroki (Reference D) found that over 80% of users did not refill their wash-bottles every day with type I ultrapure water. Horikiri’s results (Reference E) also suggested that glass bottles were preferable for storing water to plastic wash-bottles. After two days storage in each type of vessel, analysis of type I ultrapure water by LC-MS showed, among other impurities, di-n-octyl phthalate was present in the water from the wash-bottle at ppb levels but at much lower levels in the water from the glass bottle, see Figure 7. The bottom trace seen comes from type I ultrapure water injected with a mixed standard solution of six types of phthalate ester. Similar contamination problems can occur if bottled purified water for high sensitivity analysis is stored and reused after opening.
Conclusion
The extremely high purity of type I ultrapure water enables laboratory workers to obtain accurate results from high sensitivity analyses. This analytical accuracy is dependent, however, on the use of a well designed water purification system to maintain and monitor the water’s purity within the system, easy to use water dispensing and good laboratory practise during collection and use.
References
- Suzuki, Kawaguchi, Enami and Kuroki: Abstract of Proceedings of 15th Environmental Chemistry Forum, 2006, 182-183. (3)
- Clinical and Laboratory Standards Institute. Preparation and Testing of Reagent Water in the Clinical Laboratory; Approved Guideline-Fourth Edition. CLSI document C3-A4 (2006)
- Kuroki: Chromatography, 27(3), 125-9 (2006)
- Kuroki: Industrial Water, 2003, 541, 24-30. (2)
- Horikiri S., Fujita N., Kuroki Y. and Enami T. Abstracts of Proceedings of 54th Mass Spectrometry Analysis General Forum, 2006, 458-459.