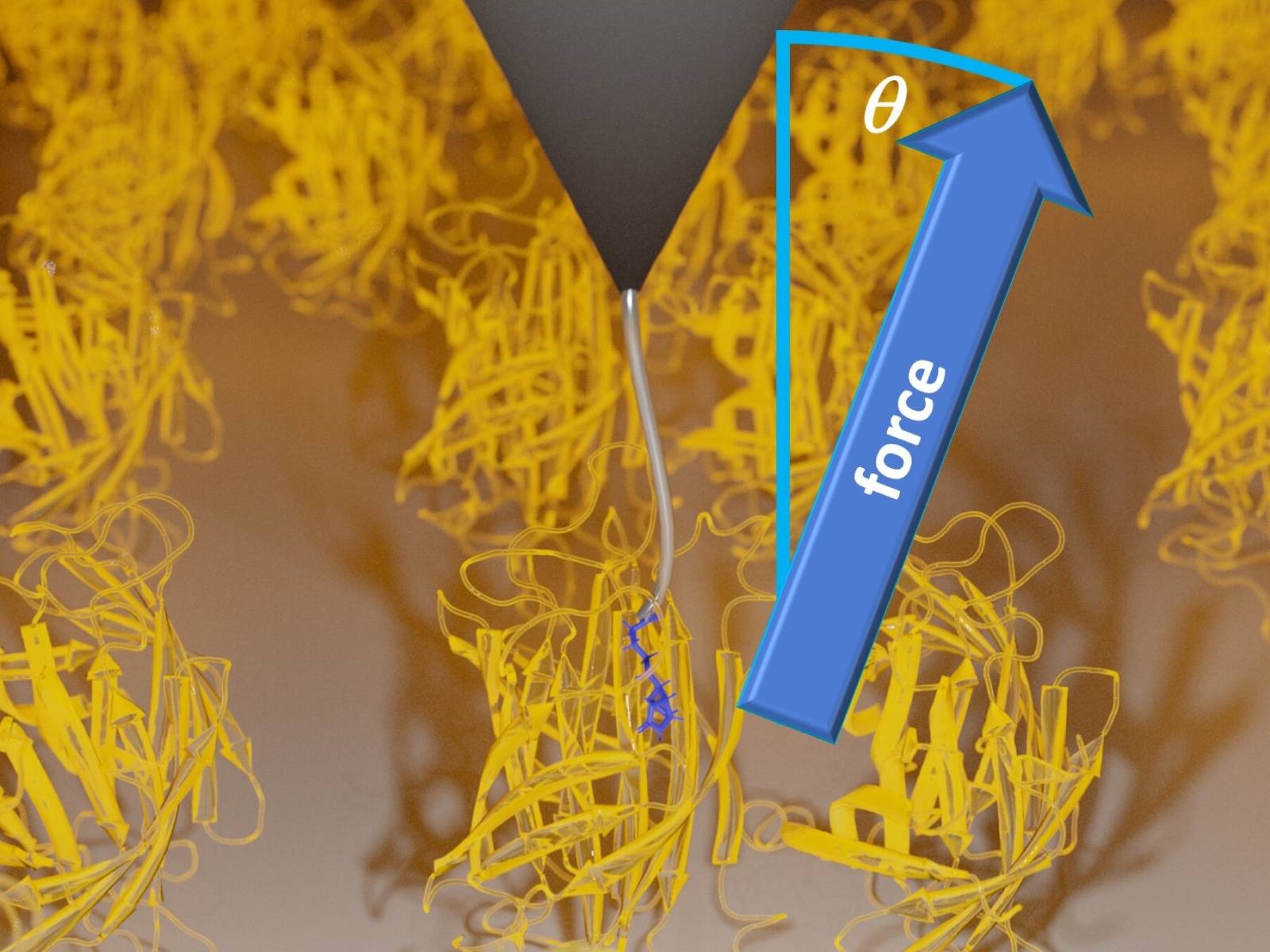
Pulling experiments on a biotin-streptavidin complex. Biotin (blue lines) is pulled out of a streptavidin protein (yellow) via a polymer linker (gray line), which is bound to a cantilever tip of an atomic force microscope (gray triangle). Credit: Dr. Steffen Wolf, University of Freiburg
The biomolecular dynamics involved in cellular activities can help scientists better understand the functions of key structures such as ligand-protein systems. While some dynamics, such as friction and heat produced from protein-internal vibrations, have been described and studied using methods like Förster resonance energy transfer (FRET), anisotropic mechanics – those that vary depending on the direction of applied forces – are not as well understood at the single-molecule level. Researchers at the University of Freiburg and the Max Planck Institute of Biophysics have now discovered a new form of friction in ligand-protein systems, using molecular dynamics simulations and advanced spectroscopy techniques to aid in their analysis.
The researchers performed experiments using the ligand-protein complex of biotin and streptavidin, applying stereographic single molecule force spectroscopy (SMFS) to collect molecular dynamics data as the protein and ligand were bound and detached by forces at different angles. In stereographic SMFS, which is based on atomic force microscopy (AFM), a single molecule of biotin is attached with a polyethylene glycol (PEG) linker to an AFM cantilever tip, which is lowered onto streptavidin molecules anchored to a glass substrate at random orientations. After the biotin binds to the streptavidin, it is detached with a pulling force – the angle of the pulling force varied between 0 (perpendicular to the glass substrate) and 75 degrees. The team then repeated the experiments in molecular dynamics simulations performed using the high performance computing resources of the BinAC HPC Cluster at the University of Tübingen.
The tests revealed that the friction that occurs during unbinding of biotin from streptavidin increases with the angle of the pulling force. This anisotropic friction, not previously identified in proteins, was determined through further computational analyses to be related to the undefinable, random orientation of the protein molecules. Thus, the researchers concluded that this type of friction is likely present in all bioassemblies in which randomly oriented proteins are met with a directional force, such as in biomolecular motors, force-sensitive membrane proteins and proteins complexes affected by the force of blood flow, explained co-corresponding author Bizan N. Balzer, a biophysicist at the University of Freiburg. This study was published in Nano Letters.
“Anisotropic friction is thus another important piece of the puzzle for understanding friction in both technical applications and in biological complexes in general,” said Balzer.